Ionic Liquids for Cleaning Operations at Air Force Logistics Centers
By Janelle Yerty, Melissa Klingenberg, Elizabeth Berman and Natasha Voevodin
INTRODUCTION
A number of industrial solvents used in cleaning operations have been designated by the U.S. Environmental Protection Agency as producing greenhouse gases (GHGs) or containing volatile organic compounds (VOCs), hazardous air pollutants (HAPs), and/or ozone-depleting substances (ODSs). Although many earlier ODS issues were addressed previously, some issues involving HAPs, VOCs, or GHGs still remain. These include concerns related to worker health and safety with cleaning operations. Ionic Liquids (ILs) have been investigated previously for coatings deposition, energy harvesting, deicing, and as green solvents for munitions applications. Recently, they have also been considered for cleaning applications (largely textile related). Because of their negligible vapor pressure, high thermal and electrochemical stability, and low melting points (less than 100 degrees Celsius (°C)), ILs offer potential as alternative cleaning agents
[1]. The toxicology of ILs depends on the specific anions and cations. For example, 1-ethyl-3-methylimidazolium chloride (EMIM [Cl]) is non-toxic, while a close derivative, 1-butyl-3-methylimidazolium chloride (BMIM [Cl]) is toxic
[2],[3].
Typically, the cation determines the viscosity and conductivity, which ultimately affects the, mass transport properties of the IL
[4]. Anion chemistry can determine the reactivity with water, coordinating ability, and hydrophobicity, though not all ILs are hydrophobic
[5]. Due to the paired nature, ILs can be designed to suit a specific need. However, the myriad of combinations also results in reduced availability, potentially high synthesis costs, and limited life cycle knowledge, which may prove to be an obstacle for using ILs as “green” solvents
[6].
A literature search and vendor survey was conducted to identify suitable IL chemistries for cleaning applications. It was found that 2-ethylhexyl lactate (2ehl) was tested as a cleaner. The published work showed that 2ehl displayed similar performance to hydrofluorinated ether (HFE) 7100, which is currently used in vapor degreasing operations at Air Logistics Centers (ALCs)
[7]. It was also found that 1-ethyl-3-methylimidazolium (EMIM) acetate, EMIM methane sulfonate, EMIM ethylsulfate, and triethylsulfonium bis(trifluoromethylsulfonyl)imide were good candidates as cleaning agents
[8].
Performance requirements for cleaning operations were derived from technical orders and military specifications supplied by ALCs paint/depaint and plating facilities
[9]-17. Cleaning efficiency, toxicity, health hazards, and materials compatibility were prioritized concerns. The ILs were largely evaluated based on environmental health and safety (EH&S) risks in terms of Health Materials Information System (HMIS) ratings and any issues indicated on the associated material safety data sheet (MSDS) and/or technical data sheet (TDS) because little to no performance data was available related to cleaning operations.
EXPERIMENTAL DETAILS
EMIM acetate, EMIM ethylsulfate, and 2ehl were selected for investigations. EMIM acetate was procured from Government Scientific Source and EMIM ethylsulfate was procured from Expotech USA, Inc. while 2ehl was procured from Purac America, Inc. Testing included an evaluation of cleaning ability and effects on metal surfaces. All testing followed industry and federal standards, including ASTM test methods, U.S. EPA standard test methods, and ALC military specification test methods, listed in Table 1.
Cleaning Efficiency Evaluation
Cleaning efficiency was conducted using pre-weighed 4130 steel and 2024-T3 aluminum (Al) panels coated with 200 milligrams (mg) of molybdenum disulfide grease (containing at least 50 mg of free soil) that were baked in an oven at 100 °C for 60 minutes (min). Five wear cycles, using each IL or dilutions thereof, were performed on each of three panels using an in-house fabricated apparatus equivalent to Gardner wear tester.
Each tested IL was diluted with nine parts water per the test specification, but also dilution ratios of 1:1, 1:2, 1:3, and the undiluted form were conducted to determine optimum performance. Percent (%) cleaning efficiency was calculated as a comparative value to that of the control solution [% by weight (wt.) d-limonene, 5% by wt. diethanolamine, 5% by wt. Triton X-100, and 60% by wt. distilled water per ASTM D 1193, Type IV]. For 2024-T3 Al, the control removed 97% of the grease, while 89% of the grease was removed from 4130 steel. Table 1 summarizes cleaning efficiency values in comparison to the control on 4130 steel and 2024-T3 substrates and associated cleanliness inspections.
Table 1. Cleaning Efficiency Tests on 4130 Steel and 2024-T3 Al
None of the 1:9 IL dilutions performed satisfactorily in comparison to the control solution. The 1:3 IL dilutions did not perform satisfactorily on 4130 steel, but were satisfactory for aluminum. Additionally, concentrated EMIM acetate dissolved the cellulose sponge and could not be tested undiluted.
Undiluted 2ehl performed the best on 2024-T3 Al and similarly on 4130 steel, with a 1:1 dilution performing only slightly better. No distinct trend was observed with increasing dilution. A 1:1 dilution ratio of EMIM acetate performed best on 4130 steel, but the 1:3 dilution ratio performed slightly better on 2024-T3 Al. EMIM ethylsulfate did not perform as well as the control on 4130 steel in any concentration; however, all concentrations, except for a 1:9 dilution ratio, performed satisfactorily on 2024-T3 Al.
Panels tested with the 1:9 dilutions also were subjected to compositional analysis using energy dispersive spectroscopy (EDS) via scanning electron microscopy (SEM) (Falcon Camscan Model# MV2300), visual examination using white and UV light, and water break tests in accordance to corresponding specification (Table 1). White light revealed minimal streaks and spotting on 4130 steel for all ILs, and UV light revealed several spots on panels treated with 2ehl. White light also showed residue, staining, and streaking on 2024-T3 specimens for all ILs, while only few to no spots were observed in the UV light. However, 2ehl-treated panels exhibited more spots and particulates under UV light. Compositional analysis gave widely varying results on 4130 steel and 2024-T3 Al, with high statistical variability within process sets. Most panels passed the water break evaluation, with only two 2024-T3 Al and one 4130 steel panel displaying water droplets using either 2ehl or EMIM acetate.
Materials Compatibility Evaluation
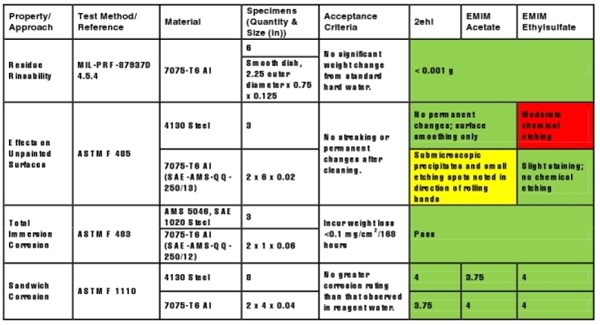
Materials compatibility testing included residue rinsability, effects on painted surfaces, total immersion corrosion, and sandwich corrosion, as is shown in Table 2. Residue rinsability testing was performed on preweighed smooth-walled Al weighing dishes filled with 25% by volume IL in standard hard water. Dishes were dried for 7.5 hours (hrs) in a convection oven at 66 °C, rinsed with distilled water for 1 min, re-dried in an oven at 66°C, and weighed using an analytical balance (Dual Range Mettler-Torledo Model# PR5003) to determine the average weight change. All three IL cleaners exhibited average weight changes that were less than the reporting limit of 0.001 grams (g), thereby passing.
Table 2. Materials Compatibility Testing
Effects of the ILs on unpainted surfaces was evaluated by immersing one end of each panel in an IL cleaner at room temperature for 5 min, removing and drying them in an oven at 66°C for 30 min, rinsing, drying, and visually evaluating each panel. On 4130 steel panels, EMIM ethylsulfate exhibited moderate chemical etching, while 2ehl and EMIM acetate only exhibited a surface cleaning effect. Conversely, on 7075-T6 Al panels, 2ehl and EMIM acetate revealed small precipitates of etching aligned in the direction of the rolling bands on the immersed Al surfaces, while EMIM ethylsulfate showed only slight staining with no etching.
The corrosivity of the IL cleaners, using total immersion testing, was also evaluated on preweighed SAE 1020 steel and 7075-T6 Al panels, by immersing three specimens of the same alloy vertically into 1000 mL glass beakers containing approximately 700 mL of each cleaner at 38°C for 7 days. Panels were removed, rinsed, and weighed after each 24-hr period. All panels in each subset exhibited an average weight loss across three panels that was less than the criteria of 0.25 mg per square centimeter (cm2) per 168 hrs for SAE 1020 steel and 0.15 mg/cm2 per 168 hrs for 7075-T6 Al.
Sandwich corrosion was evaluated on 4130 steel and 7075-T6 Al panel sets sandwiched together with a small piece of filter paper saturated in the ILs. Tested specimens, along with a control set (e.g., using filter paper saturated with de-ionized water), were placed in oven at 38°C for 8 hrs, and then in a humidity chamber (95-100% relative humidity) at 38 °C for 16 hrs each day. The cycle was repeated for 7 days. All panels passed this 1-4 rating evaluation, with corrosion not exceeding the control [i.e., rated a 4 (or less) per ASTM B117 discoloration and corrosion on more than 25% of the area].
CONCLUSIONS
Acceptable levels of cleanliness were achieved with 2ehl, and materials compatibility testing for this cleaner showed that the corrosivity of the chemistry was acceptable on both aluminum and 4130 steel. Likewise, minimal residue (as compared to that of standard hard water) remained on aluminum weighing dishes in residue rinsability testing of 2ehl. Its effects on unpainted 4130 steel surfaces were minimal (i.e., no permanent changes detected), but etching in the direction of the aluminum rolling bands was detected on 7075-T6.
EMIM acetate performed similarly to 2ehl, with the only notable difference being a slight improvement in cleaning efficiency.
EMIM ethylsulfate demonstrated lower cleaning efficiency values than 2ehl and EMIM acetate on 4130 steel, but displayed similar results to the other tested ILs on 2024-T3 aluminum. Similarly, the corrosivity results were similar to the other tested ILs as was residue rinsability. However, its effects on unpainted surfaces was very different from 2ehl and EMIM acetate in that it did not etch 7075-T6 and only produced minimal staining, but produced moderate chemical etching on 4130 steel.
Because chemical etching was noted on 4130 steel and 2024-T3 aluminum for each IL tested, additional investigations should be conducted to determine whether the etching noted by all three ILs will adversely impact the mechanical properties of the substrates being cleaned. Future work will include hydrogen re-embrittlement testing to ensure that the ILs do not affect the integrity of the substrate. Additionally, chemical evaluations of the ILs will be conducted to ensure that it meets military specification requirements.
Janelle Yerty and Melissa Klingenberg, Ph.D., Concurrent Technologies Corporation (CTC)
Elizabeth Berman, Ph.D., Air Force Research Laboratory (AFRL)
Natasha Voevodin, Ph.D., University of Dayton Research Institute (UDRI/AFRL)
[1] Abedin, Sherif Zein El, and Frank Endres, “Ionic Liquids: The Link to High-Temperature Molten Salts,” Accounts of Chemical Research, Nov. 2007.
[2] Basionics, 1-Ethyl-3-Methylimidazolium Chloride (CAS No. 65039-09-0) Data Sheet, BASF Group, 2010.
[3] Basionics, 1-Butyl-3-Methylimidazolium Chloride (CAS No. 79917-90-1) Data Sheet, BASF Group, 2010.
[4] Abbott, Andrew P. and Katy J. McKenzie, “Application of Ionic Liquids to the Electrodeposition of Metals,”
Phys. Chem. Chem. Phys., 2006, 8
, 4265-4279.
[5] Rogers, R., et.al., “In Ionic Liquids as Green Solvents,” ACS Symposium Series, American Chemical Society; Washington, DC, 2003.
[6] Holbrey, John, Megan B. Turner, and Robin D. Rogers, “Ionic Liquids as Green Solvents: Chapter 1: Selection of Ionic Liquids for Green Chemical Applications,”
American Chemical Society Symposium Series, 2003.
[7]National Aeronautics and Space Administration (NASA) Final Report and Deliverables, “Precision Cleaning of Oxygen Systems and Components,” NASA/CR-2009-214757.
[8]Dr. Markus Wagner, MERCK; Dr. Megan O’Meara and Dr. Aurelie Alemany, BASF Corporation; Dr. Khalid Shukri and Dr. Andrew Abbott, Scionix, telephone correspondence, November 2010.
[9]Technical Order TO 42C2-1-7, “Technical Manual: Process Instructions: Metal Treatments: Electrodeposition of Metals and Metal Surface Treatments to Meet Air Force Maintenance Requirements,” 28 Feb. 2006.
10Boeing Specification BAC-5408, “Vapor Degreasing, Revision M.”
11Boeing Specification BAC-5765, “Cleaning and Deoxidizing of Aluminum Alloys.”
12Boeing Specification BAC-5555, “Phosphoric Acid Anodizing of Aluminum for Structural Bonding.
13Military Specification MIL-PRF-87937, “Performance Specification, Cleaning Compound, Aerospace Equipment, 24 Sept. 2001.
14 Military Specification MIL-PRF-680, “Performance Specification, Degreasing Solvent,” 26 Oct. 2006.
15Technical Order TO 1-1-691, “Cleaning and Corrosion Prevention and Control, Aerospace Equipment and Non-aerospace equipment, 19 Oct. 2007.
16 Technical Order TO 2-1-111, “Standard Maintenance Procedures, Navy and USAF, P&W Aircraft Engines.”
17 Technical Order TO 1-1-8, “Application and Removal of Organic Coatings, Aerospace and Non-aerospace Equipment,” 12 Jan. 2010.
RELATED CONTENT
-
While the red color may not be desirable, anodizing expert Drew Nosti says it poses no particular problem to a successful anodizing process.
-
In this paper, a review of several process solutions, examining coolants, solvent cleaning, alkaline clean/etch and deoxidizing/desmutting, listing intended and unintended chemical reactions along with possible mechanisms that would favor corrosion formation.
-
This important first step can help prepare the metal for subsequent surface finishing.