Calculating Corrosion
Advancement in the control and prediction of CASS test performance...
The demand for improved corrosion performance from automotive decorative chromium components has evolved during the last 20 years. This has generally been characterized by the extended CASS (Copper Accelerated Acetic Acid Salt Spray) test requirement in automotive standards. CASS test performance is strongly linked to microporosity, among other deposit attributes.
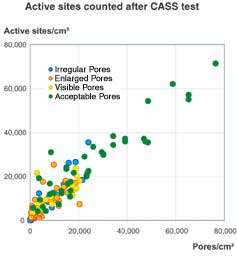
Recently, one automotive manufacturer adopted a new method for assessing active micropores using the Fuhrmann test instead of the Dubpernell test. The advantage of the new procedure is the direct correlation of the pore count before CASS test to the active sites after CASS exposure. Consequently, the Fuhrmann test is effective for predicting corrosion results, assuming all other deposit characteristics such as thickness and STEP (Standard Test Method for Simultaneous Thickness and Electrode Potential Determination of Individual Layers in Multilayer Nickel Deposit) comply with the specification. The acceptance of the Fuhrmann test by other OEMs is anticipated due to its improved reliability.
Featured Content
Electroplated metal and plastic components are used extensively in some of the most severe corrosive environments, such as the exterior trim of cars. Because of this, automotive designers demand a stain- and defect-free appearance and exceptional corrosion resistance. Quickly verifying the corrosion performance of the surface finish presents an advantage to both the production plating plant and to the end user. The combination of a controlled microporous nickel chromium process, the STEP test and the Fuhrman porosity test procedure provides the key elements for a reliable decorative system.
Decorative Chromium Plating
Bright chromium plating has always been the favored decorative plated finish for both metal and plastic components. Chromium is completely tarnish resistant, hard, and wear resistant, with an elegant blue-metallic appearance. Bright chromium is not just a single chromium layer. It invariably has one or more under layers of electroplated nickel, ranging from 5–50 micrometers thick. The visible top layer is a thin chromium coating, typically with a thickness of 0.4 micrometer.
For components used indoor, such as office furniture, office equipment and domestic appliances, a single layer of bright nickel is adequate to afford the necessary surface improvement. This, combined with the chromium layer, provides sufficient product durability. At the other end of the environment spectrum, exterior automotive trim presents much greater demand on the metallic layers in terms of corrosion resistance, and, subsequently, it requires a more complex system.
Research going back 30 years or more has shown that significant improvements in corrosion resistance can be achieved with two or more different nickel layers and special microdiscontinuous chromium top-coating or strike layer.1 Today, multi-layer nickel and microporous chromium are the preferred choice to resist even the most severe environments.
Corrosion attack on nickel chromium systems occurs at discontinuities in the chromium layer. A corrosion cell is set up with the chromium as the cathode and the nickel as the anode. When there are a few defects in the chromium layer, the surface area of the cathodic chromium is greater than the surface area of the anodic nickel. A corrosion cell forms, and nickel is dissolved rapidly, resulting in visible corrosion.
Microporous Chromium
Simple chromium layers have random discontinuities such as cracks or pores. In practice, these defects are difficult or impossible to eliminate, so corrosion occurs at these points. The solution to this has been to produce many discontinuities in the form of cracks or pores in the chromium layer.
A microdiscontinuous chromium layer over duplex nickel layers provided a major improvement in corrosion resistance and met end user requirements from the 1970s until the mid-1990s.2 During this period, extensive accelerated testing and service life evaluations demonstrated that microporous chromium was the preferred method of producing microdiscontinuous chromium. In recent years, microporous chromium has been recognized by European automotive manufacturers, where microcrack chromium was more widely accepted.3
The revival of decorative chromium for automotive trim in the mid-1990s pushed demand to new heights, stimulating development and refinement of techniques and processes. Research focused on the critical areas of consistency, control and rapid evaluation of production quality.
To produce microporous chromium, a special diphase bright nickel layer is deposited directly before the chromium. This special nickel process contains very small, inert particles co-deposited with the bright nickel. Particle selection is based on an optimum range in size or diameter. The particles protrude from the nickel layer and interrupt the deposition of chromium layer.
Consequently, particle distribution, particle size and corrosion activity of the pores are heavily dependent on the co-deposition mechanism of the inert particles. A uniform particle distribution over the surface of complex-shaped components is necessary for corrosion protection on the entire part.
Duplex Nickel
To control corrosion and provide optimum base material protection, different nickel plating processes are used to produce the multi-nickel layers. Semi-bright nickel is plated first, followed by bright nickel.
Semi-bright nickel is a purer layer with a different structure from bright nickel. The purity is characterized by the low sulfur content in the layer. Semi-bright nickel must have less than 0.005% sulfur, compared to bright nickel, which typically contains 0.04% sulfur.
The significance of the sulfur is directly related to the corrosion of the layers. In a corrosion cell, the semi-bright nickel is cathodic to the bright nickel, so attack or dissolution is confined to the bright nickel layer. The semi-bright nickel layer remains intact and provides protection for the substrate.
Solution additives and electrolyte purity control the activity of the layers. Proper maintenance of the nickel electrolytes is one of the key factors in optimizing the corrosion resistance of the system.
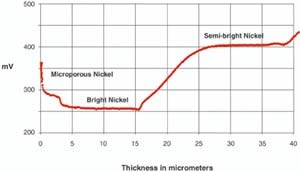
The STEP test was developed at the Chrysler Corporation for measuring the thickness and the dissolution potential of nickel layers.4
Figure 1 illustrates the dissolution of the nickel layers and simultaneous thickness measurements of the deposit. Also, the microporous layer is more noble, or inactive, than the bright nickel. This is an advantage in a corrosion cell, since the bright nickel corrodes preferentially, maintaining the chromium and microporous nickel layer intact. The component remains untouched even after prolonged exposure to the most severe environment. Likewise, the semi-bright nickel is considerably more noble than the bright nickel and corrodes preferentially and sacrificially, protecting the substrate.
Extensive observation of service performance has shown a clear correlation between an appropriate potential difference or correct STEP and exceptional durability.5 The STEP test is now incorporated into most automotive specifications.6
Quality Control
A dilemma faced by every quality manager is ensuring that the product coming off the plating line meets the desired quality standards. Most quality control methods for electroplated parts involve lengthy corrosion testing, such as the CASS test. At times, up to 100 hours may be required. The STEP test is the exception, requiring only a few minutes; although, alone, it is insufficient to indicate the corrosion resistance of the system.
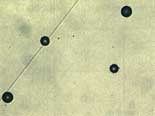
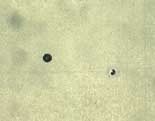
An equally critical factor is the number of active pores in the microporous chromium, since this has a major influence on maintaining the surface appearance after corrosion. The Dubpernell test has been used for many years. This method uses the deposition of copper on the plated component. Since copper does not deposit on the chromium, only the exposed nickel is plated with copper, which is an indication of microporosity.
When the typical automotive specification only required 16–24 hours of CASS testing, the Dubpernell test was adequate to indicate subsequent performance in the accelerated corrosion test. However, as quality requirements were extended to 48 hours CASS and above, it became clear that the pores indicated by the Dubpernell test were not always active after exposure to the CASS test. It was not uncommon to find failures on components, which appeared to have the correct number of pores based on the Dubpernell test.
Recent research has produced an improved pore count test known as the Fuhrman test. This method is based on the Dubpernell method, but the deposition of the copper is carried out at a prescribed voltage setting on a defined surface area. Variations in current, common to the Dubpernell test are eliminated. Only pores capable of creating corrosion cells or active sites are visualized. Consequently, there is a direct correlation between the Fuhrmann test pore count and the number of active sites after CASS testing. Both DaimlerChrysler and Volkswagen have included the Fuhrmann test in their specifications.7
Clearly the aesthetic appeal of decorative chromium trim has contributed to its popularity on automobiles for more than 30 years. In addition to its bright appearance, consistent reliable corrosion performance will ensure its continued use in the future. The combination of a controlled microporous nickel chromium system, the established STEP test, plus the new Fuhrmann test provides the plating suppliers a practical means to verify and predict CASS test corrosion results. The advantage is rapid feedback on the integrity of the finished product to meet the automotive manufacturers’ standards.
RELATED CONTENT
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
-
Stripping of Plated Finishes
The processes, chemicals and equipment, plus control and troubleshooting.
-
Cleaning, Pretreatment to Meet Medical Specs ISO 13485 or FDA 21 CFR820
Maximilian Kessler from SurTec explains new practices for industrial parts cleaning, metal pretreatment and decorative electroplating in the medical device industry.