Written by:
Jude M. Runge, Ph.D.,
CompCote International, Inc., Elmhurst, IL,
Thomas Nussbaum,
Compcote International, Inc. Elmhurst, IL,
Richard Rosenfield,
Douglas Alexandria Finishing, Inc., Alexandria, MN
Presented at the 19th Annual International Anodizing Conference & Exposition, October 5-7, 2010, Montreal, Quebec, Canada. For more information, please visit Anodizing.org
R. Buckminster Fuller (1895 – 1983), one of the greatest American thinkers and inventors of the 20th century and a visionary for the 21st, once said, “A designer is the emerging synthesis of artist, inventor, mechanic, objective economist and evolutionary strategist.” 1 Evaluation of these elements reveal the synergy of creativity, technological know-how, business savvy and forward thinking needed to design something new or to modify existing designs. If one or more of the driving elements shift, designs must change or suffer obsolescence. When the economy changes, which is one of these driving elements of design according to Fuller’s definition, opportunity knocks because existing designs need to be modified, for example, for cost reduction purposes.
Many industries that require innovative solutions in cost reduction and weight savings are turning to aluminum as a substitute for stainless steel and other steel alloys for parts and components. Aluminum is lighter weight, and many aluminum alloys exhibit high strength to weight ratios which in many applications enable the replacement of stainless steel with considerable weight savings. Furthermore, aluminum is recyclable, and less expensive in both material and fabrication. It is easier to process, and many aluminum extrusions and castings can be produced near net shape, which saves time and money in post-process machining.
The main difference between stainless steel and aluminum, other than cost and weight, is in the inherent corrosion resistance of stainless steel. In wear applications, stainless steel must be finished, nickel plating or specialized carburization processes are the typical methods of choice. However, aluminum and aluminum alloys exhibit long term corrosion and wear resistance when anodized; a process both less expensive and more environmentally friendly. Awareness of market trends and their influence on the elements of design brings new business opportunities for the metal finisher.
Designing with Aluminum in Mind
By approaching component design following Buckminster Fuller’s definition, a clear rationale for the substitution of stainless steel with appropriate aluminum alloys is made.
Design as an Art
Aluminum is the material of choice for stainless substitution first because of its appearance: it has the same light silver color. The beauty and variety of colors that one can achieve with anodizing aluminum is possibly the most obvious design use of aluminum as art. See figure no. 1.
Color can be used to decorate, but can also be used to differentiate, as in applications in the medical equipment industry that require mock-ups for quick sizing (as in medical try-ins) or for component differentiation (kit boxes for medical instruments) or measurement applications (dental probes for periodontic applications). See figure no. 2.
Many industries seek value in appearance. Brushed stainless steel kitchen appliances and stereo components have set an appearance standard that is easy to maintain. Anodized brushed aluminum is an excellent substitute for brushed stainless steel because of its inherent color and because it brings the advantage of custom colors. There is a disadvantage: many cleaners are alkaline based which limits the appearance lifetime of the anodic oxide finish. This can be overcome with customer warnings on such products as a true alkaline-resistant anodic oxide finish has yet to be developed.
The design input of the metal finisher as to the decorative capability of the anodic oxide finish is imperative in these applications and can be the driver of change within the framework of design use of materials. Limitations such as finish alkaline resistance show a need for more research and development in this area and many programs are currently underway.
Design as an Invention
Fundamental concepts that evolve into invention stem from the need to solve a problem, and the solution must be novel for the result to be considered an invention. A material change is typically enough to be considered innovative, especially when the corresponding design elements show advantages because of the change. Hence, taking components that were once made from stainless steel and substituting aluminum alloy as the basis material, when the result is functional, can be considered invention.
Design as a Mechanic
For the purpose of this paper, and for the purpose of expanding on Fuller’s definition of design, the element of design as mechanic is expanded to encompass the total application; specifically, for the purpose of addressing corrosion resistance. Without the design consideration of the application environment, the mechanical performance of the design will ultimately suffer.
Steels and cast irons have been widely used for engineering applications because of their strength and hardness coupled with other characteristics. Stainless steel alloys have been used for their strength but in particular because of their corrosion resistance in many severe environments. In wear applications, stainless steel must be finished, nickel plating or specialized carburization processes are the typical methods of choice. Furthermore, stainless steel has shown a level of biocompatibility (corrosion resistance in the biological milieu) that made it the material of choice for dental and surgical implants, medical instruments and other equipment utilized for biomedical applications.
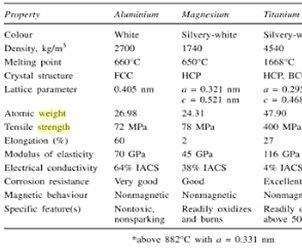
Beginning with World War II, when transportation of all types took on dire importance, interest in the development of low density alloys with good strength-to-weight ratios was strongly felt. Aluminum, beryllium, magnesium and titanium, having low densities, were given a great deal of attention. Beryllium was eventually discarded as a base metal due to its expense and limitations were quickly found with magnesium because it was difficult to make magnesium alloys and mechanical working was difficult. In addition, there are many different qualities of aluminum and titanium that make them useful engineering metals. A summary of the properties of the light metals is provided as Table I.2
Low density materials have become the greatest asset to the transportation industry, enabling the reduction of mass of air, sea and road vehicles which in turn increase speed and payload capacity while reducing fuel consumption. Sporting goods such as bicycles and sailboats have also shifted to low density materials for these same reasons. The principle criterion for this replacement is the specific strength of aluminum and titanium which is determined by their strength-to-weight ratios. Strength can be identified by yield strength, tensile strength or elastic modulus, depending upon application.3 Table 2 lists the yield strength values for some aluminum alloys with common tempers and stainless steel alloys with normal and low (L) carbon values.4, 5
In addition to high strength-to-weight ratio; aluminum boasts good ductility and malleability, excellent formability and manufacturability, high corrosion resistance and very good mechanical properties at subnormal temperatures. Titanium, while exhibiting a high strength-to-weight ratio, cannot compete with the ease of formability and manufacturability of aluminum.
As an element, aluminum exhibits a rather low tensile strength, but this is increased by alloying. The added strength of the aluminum is derived in two ways: by solid solution hardening and/or by precipitation hardening. Although alloying increases strength, it decreases corrosion resistance. Therefore, design must take into consideration the possibility of a surface finish.
Aluminum and stainless steel share the common mechanism of passivity as the primary source for corrosion resistance. Stainless steel can be passivated to enhance its corrosion resistance much like aluminum is conversion coated. However, the structure of the basic passive finish on stainless steel is far different than that of the basic passive finish on aluminum as the passivity of stainless steel is often related to the alloy chromium content while aluminum passivity is based upon the chemical reactivity of aluminum with oxygen.
The oxide finish on aluminum can be enhanced electrochemically and grow into a functional oxide by way of anodizing, which has an appreciable thickness, and imparts decoration, corrosion resistance and wear resistance depending upon the finishing process. The characteristic of aluminum to be anodized to form an integrated oxide finish separates it from stainless steel; an integrated finish does not typically exhibit the propensity to fail by way of delamination.
Design as an Objective Economist
Upon consideration of the two primary light metals used in industry, titanium is a high cost choice of material for both raw material and processing. This fact limits the use of titanium to specialty applications, and increases the opportunity for the use of aluminum in appropriate applications that may have considered titanium, but balk because of the high cost.
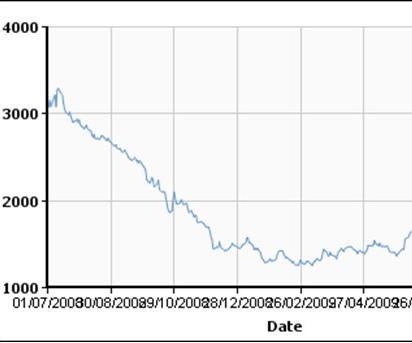
With respect to stainless steel, in interviews with three companies serving three different industries, the motivation for design change was clear: cost reduction.6, 7, 8 Stainless steel is expensive. In August 2008, shortly before the crash of the financial system and the downturn of the economy, the global all products stainless steel price for grade 304 was about 4,300 US $/ton (Meps, UK), the price for aluminum ( 99.7% pure) was about 3,300 US $/ton (LME). Comparing these values yields a price ratio between stainless steel and Aluminum of 1.3 to 1.0. In August 2009, stainless steel cost about 2,800 US $/ton, whereas Aluminum cost 1,900 US $/ton.9, 10 Comparing these values yields a price ratio of 1.47 to 1.0. See Tables 2 and 3.
Stainless steel is about 3 times heavier than aluminum. The product of the weight ratio (3) and the effective price difference for the same volume of material between stainless steel and aluminum in August 2009 (1.47) is 4.41. These price trends show that for the same volume of material, stainless steel is more than 4 times more expensive than aluminum.
Aluminum is also less expensive in fabrication. It is easier to process than stainless steel and can be easily and inexpensively cast, and otherwise wrought through rolling, extrusion, deep drawing, and blanked and formed to near net shape. Cost savings can therefore be realized in the reduction of finish machining.
Wear resistance of stainless steel is often increased through the use of nickel plating (electroless and electrolytic) as well as by specialized heat treatments that carburize the outer surface (e.g. Kolsterizing). While processes such as these are effective, and often impart an increased level of corrosion resistance, they nevertheless add expense. As a deposit, nickel plating can blister, flake and delaminate. There are significant environmental ramifications with the use of nickel, particularly in waste treatment and disposal also must be considered as they add cost.
The ultimate money saver is time. By substituting stainless steel with the appropriate aluminum alloy, fabrication and process time are also reduced. Higher product volumes at lower cost are realized faster.
Design as an Evolutionary Strategist
What is an Evolutionary Strategist? This element of design encompasses forward thinking – imagining scenarios that consider the role of the design in the future. These strategic considerations are necessary to develop designs that will not be obsolete when they are produced and exceed the relationship of the design with respect to its application in business. These strategic considerations must also address the relationship of the design with respect to its application in the world.
When considering the various attributes of aluminum in today’s global political climate, what is “green” or good for the environment comes directly to the front of one’s mind. Aluminum and aluminum alloys exhibit a number of advantages that not only bring cost savings because of ease of processing and decreased time-to-market; these advantages also save energy which is important for the environment. In addition, because of weight savings, its incorporation into designs enables larger pay loads and saves fuel.
Possibly the most obvious advantage with aluminum and aluminum alloys is that they are recyclable, even when anodized. Recycling is a must because the process for extraction from bauxite consumes high amounts of energy. The biggest concern with the recycling of aluminum alloys is in reprocessing the recycled material.
Trace elements, the smaller alloy constituents added to refine the microstructure and enhance mechanical properties, are often higher in recycled product, although within the specified tolerances for the alloy. Reprocessing must include rapid solidification in order to produce metal that can tolerate the higher trace element content as rapid solidification hinders the formation of precipitates that will coarsen and migrate to grain boundaries with time at higher temperatures. Slow-cooled or inefficiently cooled product exhibits precipitates and intermetallic compounds that produce streaking and discoloration through subsequent wrought processes as well as in anodizing.
Actual Industrial Examples of Successful Substitution with Aluminum
Accessories
Metal gives the weight and appearance, the overall feeling of value. For many applications; custom jewelry, small accessories and electronic devices, metallized plastic substituted stainless steel. Many manufacturers of these components are returning in greater numbers to aluminum for the value of this important feeling of value. This is often the case for higher end accessories. See figure no. 3.
The volumes and low cost requirement for these components often drives the business off-shore to Asia. Herein lays the limits of some applications: often off-shore foundries only cast or process one aluminum alloy, most commonly alloy 380, which does not yield an aesthetically pleasing finish when anodized, especially when the desire is the look of stainless steel. In these cases, the design input of the metal finisher as to the decorative capability of the anodic oxide finish is imperative in these applications; for example, to recommend a different alloy eg. ADC 6, and/or a different pretreatment, such that the oxide can provide the desired appearance. In this role, the metal finisher can be the driver of change within the framework of the component design.
Home Appliances
As mentioned previously, the house wares industry, in particular, kitchen appliances is replacing brushed stainless steel with brushed clear-anodized aluminum. The look is virtually identical and is far less expensive in basic material costs and fabrication. Limitations are in the alkaline resistance of the anodized finish.
Another application can be found in bathroom fixtures. Due to the expense of copper and copper alloys, the manufacturers of bathroom faucets, shower heads and other bath accessories is also turning to aluminum, and for decorative reasons, anodizing is the finish of choice. Extensive testing and analysis has gone forward to document there is no aluminum migration into the water, even at high temperatures. Anodizing gives bathroom designers the opportunity of beautiful colors, and the metal finishing industry needs to be ready to fulfill this opportunity. See figure no. 4.
Medical
Stainless steel has shown a level of biocompatibility (corrosion resistance in the biological milieu) that made it the material of choice until the early 1980’s for dental and surgical implants, medical instruments and other equipment utilized for biomedical applications. The biocompatibility of titanium coupled with its high strength-to-weight ratio has since made it the material of choice for medical implants; however problems with manufacturability, difficulty with casting and base metal expense has limited its application mainly to implantable devices.
Although aluminum cannot claim the level of biocompatibility of titanium or stainless steel and therefore cannot be used in implantable prostheses or other implantable devices, it has proven itself a valuable substitute in applications such as external prosthetic devices, surgical try-ins, instruments and other tools. See figure no. 5.
Metal finishing for the medical industry has many requirements that stem from the need to sterilize, often through steam autoclaving or other cold sterilization processes that use aggressive biocides. Stainless steel hand instruments have shown limitations to these sterilization processes in the development of red rust on cold worked handles during steam autoclaving. This reaction is due to the ferrite conversion that can occur through cold work with Austenitic (300 series) stainless steel and, of course, doesn’t occur with aluminum. However, dye migration, fading or bleed out can occur from anodized and dyed aluminum instruments and other devices. The metal finisher who engages with this industry must be ready to scrutinize the anodizing process from start to finish, with particular attention to dyeing and sealing operations.
As with other industries that utilize small components and accessories, the medical industry sometimes opts for less expensive plastic for non-invasive applications. Plasticizers used in plastics to maintain toughness can migrate during sterilization and lead to embrittlement of these instruments and tools. But to the advantage of the metal finisher, doctors, dentists and surgeons prefer the weight and appearance, the overall feeling of value that comes with using metal tools.
Machine Building
Complex and precise machines that serve a variety of industries have typically used finished carbon steel components typically surface treated by painting or nickel plating, or stainless steel components as strength requirements and in many applications, wear and corrosion resistance requirements were fulfilled by using these materials. With cost reduction as the driving force, and weight savings a close second, the use of aluminum for various types of machine components has become more prevalent in recent years.
Cost savings in substituting stainless steel with aluminum is twofold in material and fabrication time. The ability to extrude complex aluminum shapes to net or near-net shape using typically very inexpensive tooling is another big plus. The cost to purchase aluminum extrusion dies to make multiple prototype parts in the desired shape is roughly the same cost as making one approximate part via an assemblage of stainless steel machined components. In comparison, stainless steel extrusions are nowhere near net shape and require extensive post-extrusion machining. By using anodized aluminum for machine components that require wear resistance, nickel plating and/or expensive post machine carburization heat treatment is removed from the machine, saving process cost and added cost from environmental restrictions.
For the two companies interviewed that build machines for packaging and for paper applications, the cost savings ranged from 10 - 35% for components manufactured from aluminum alloy 6061 and 6005A. Yield strength was the strength value used to determine the strength-to-weight ratio, and both 6061-T6 and 6005A-T651 aluminum alloys have a yield strength value of 35 ksi. 316 stainless steel also has a yield strength of 35 ksi, making the aluminum alloys attractive replacements. Weight savings for components was up to 60% less than the corresponding component manufactured from 316 stainless steel.
Corrosion resistance of the 6000 series is higher than that for the aircraft grades of aluminum (2000 and 7000 series alloys) which made 6000 series aluminum alloys the material of choice, in particular alloy 6061, especially in the paper application. Smaller components that were once machined from 316 stainless steel bar are now being considered for die cast aluminum alloy. Both companies are using anodizing as their finishing process of choice and use Type III anodic oxide and composite Type II anodic oxide as their finish of choice for the packaging machine and paper machine applications respectively.
Both companies use a Material Review Board as part of the design process. All components on the machines, made from plated carbon steel or stainless steel are being scrutinized for replacement with anodized aluminum alloy. Regardless of anodic oxide finish type (II or III) the finish is dyed to aid in differentiation between the aluminum and stainless steel and steel alloy components and to add corporate identity to the machines. See figure no. 7
Conclusion
By approaching component design following Buckminster Fuller’s definition, a clear rationale for the substitution of stainless steel with appropriate aluminum alloys is made.
Aluminum and aluminum alloys have found more application in many industries worldwide. Cost savings in material cost and fabrication, time to market and interesting, effective, inexpensive surface treatments such as anodizing continue to develop new markets for aluminum and its alloys as substitutes for carbon steel and stainless steel alloys.
In most cases, an aluminum alloy base material can be found that has the strength and hardness for applications that typically use carbon steel and stainless steel alloys. There are a variety of anodizing processes and types that may be considered for the myriad of applications that require corrosion and wear protection. Applications testing may be necessary to determine if the material substitution as well as the finish of choice is appropriate.
It is necessary that the metal finisher be aware of the trend to substitute other materials with aluminum and therefore to explore possibilities and opportunities to serve these new markets. Awareness, however, is not everything. The producers and suppliers of aluminum and aluminum semis are more aware of who is purchasing aluminum and have a vested interest in promoting this trend to substitute. Therefore, metal finishers concerned with the future of our industry, our professional associations involved with aluminum products and the aluminum companies initiate a joint program to promote aluminum designs and products to OEM companies and their suppliers, as well as to the engineering schools who educate future designers.
The role of the metal finisher in design can be expanded by attempting to work more closely with customers. Ask why more. Many manufacturers have as part of their cost reduction programs a Material Review Board which reviews parts and components – ask to participate. Be ready to suggest realistic substitutions that fulfill your customers’ requirements when the component is anodized. These suggestions can be cost saving and expand opportunity for increasing business.
References:
- 1. Buckminster Fuller: Starting with the Universe, Exhibition, Museum of Contemporary Art, Chicago, March 14 – July 5, 2009.
- 2. Engineering Materials, Properties and Applications of Metals and Alloys, C.P. Sharma, Prentice-Hall of India, New Dehli, 2004.
- 3. Aluminium Taschenbuch, Aluminium Verlag GmbH, Düsseldorf, 1984.
- 4. Aluminum and Aluminum Alloys, J.R. Davis, editor, ASM International Handbook, Materials Park, OH, 1993.
- 5. Efunda Website, “Efunda – The Ultimate Online Reference for Engineers”, www.efunda.com.
- 6. Interview, Mr. Richard Rosenfield, Douglas Alexandria Finishing Co., Alexandria Minnesota.
- 7. Interview, Name withheld.
- 8. Interview, Name withheld.
- 9. Meps (International) Ltd., Independent Steel Industry Analysts, www.meps.co.uk.
- 10. London Metal Exchange, Exchange for Nonferrous Base Metals, www.lme.co.uk.
RELATED CONTENT
-
While the red color may not be desirable, anodizing expert Drew Nosti says it poses no particular problem to a successful anodizing process.
-
In this paper, a review of several process solutions, examining coolants, solvent cleaning, alkaline clean/etch and deoxidizing/desmutting, listing intended and unintended chemical reactions along with possible mechanisms that would favor corrosion formation.
-
Types of anodizing, processes, equipment selection and tank construction.