Development of Ni-Based High Wear Resistance Composite Coatings
Final Report - AESF Research Project #R-116
Hard chromium has been used in automotive, aerospace, mining and general engineering industries due to its excellent wear resistance and low coefficient of friction.
#surfin #aerospace #automotive
Hard chromium has been used in automotive, aerospace, mining and general engineering industries due to its excellent wear resistance and low coefficient of friction. However, hexavalent chromium-based coatings are under severe regulations owing to their toxicity and research and development of alternate coatings are in progress.1 Ni-P and Ni-B graded coatings have been proposed as possible replacement coatings for the existing hard chromium coatings.
Engineering components are subject to failure through surface degradation processes such as wear, oxidation, corrosion and fatigue under varied circumstances. Different techniques, such as physical vapor deposition (PVD), chemical vapor deposition (CVD), etc., are widely used for engineering the surface to impart desirable mechanical properties. Electrodeposition and electroless plating processes have received widespread acceptance owing to their less complex nature and cost-effectiveness. Alloying of phosphorus or boron along with nickel has improved hardness, corrosion resistance and wear resistance.2-5
- To develop a galvanostatic and potentiostatic DC and pulse plating method for the deposition of environmentally-benign Ni-P and Ni-B composite coatings.
- To study the deposition process as a function of parameters such as electrolyte composition, deposition current density/applied potential, deposition temperature and electrolyte agitation conditions.
- To determine the effect of the concentration of the particulates such as organic additives in the solution on the deposition process and mechanical properties.
- To study the mechanical and corrosion properties of Ni-P and Ni-B coatings.
- To prepare Ni-P-X and Ni-Zn-X (X=SiO2) composite coatings and to study their corrosion and mechanical properties including wear resistance, hardness, coefficient of friction etc.
Bath constituents | Ni-P |
NiSO4•6H2O NiCl2 H3PO3 H3PO4 Citric acid pH Temperature (°C) Current density (mA/cm2) | 100 - 195 g/L 2 - 13 g/L 3.5 - 24.8 g/L 1.8 - 23.6 ml/L 45 - 125 g/L 3.25 60 20 |
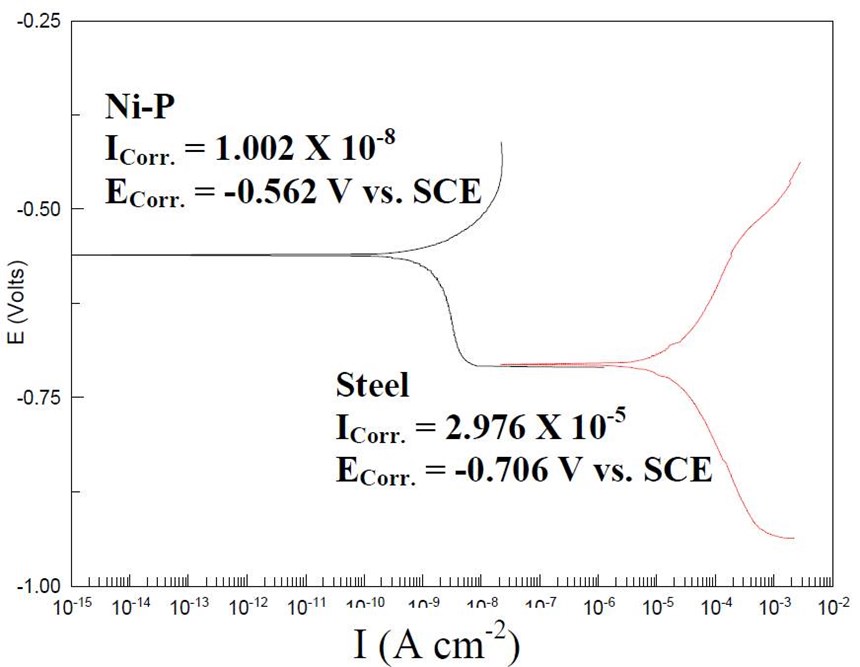
Coating | Corrosion potential (VSCE) | Corrosion current density (A/cm2) | Corrosion rate (mpy) | Hardness (HV100) |
Steel | -0.706 | 2.97 × 10-5 | 13.57 | 141 |
Ni-P | -0.562 | 1.002 × 10-8 | 0.0044 | 173 |
Table 3 - Electroplating bath composition for preparing Ni-B deposits.
Bath constituents | Ni-P |
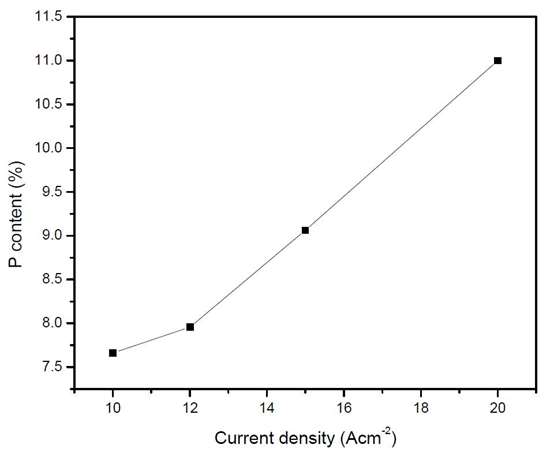
NiSO4•6H2O NiCl2 Sodium borohydride Citric acid pH Temperature (°C) Current density (mA/cm2) | 75 - 165 g/L 28 -52 g/L 6.8 - 9.2 g/L 115 - 125 g/L 2.0 60 ± 5 10 - 20 |
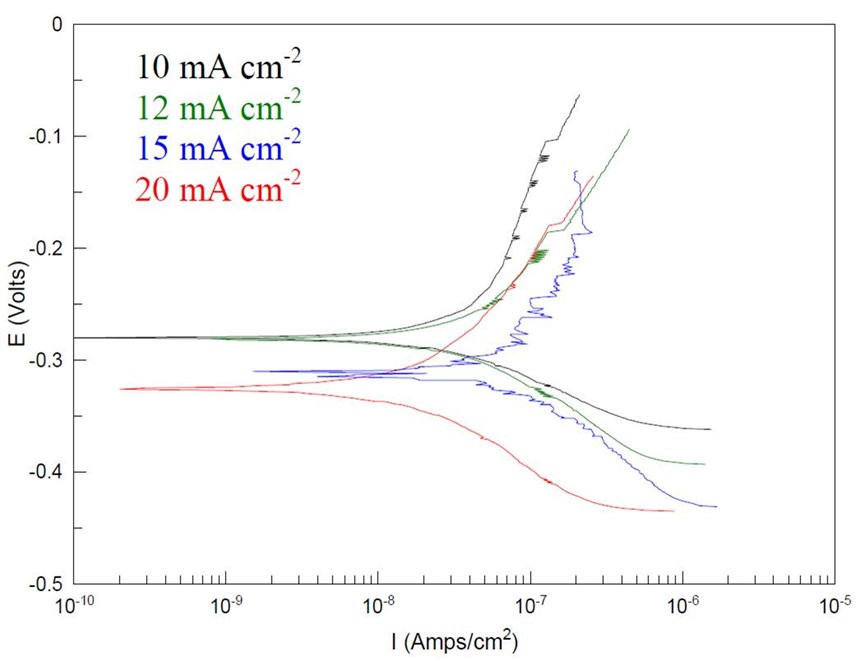
Ni-B | LP (Ω/cm2) | Icorr (A/cm2) | Ecorr (VSCE) | Βa (mV) | Βc (mV) |
10 mA | 1.24×105 | 1.31×10-8 | -0.280 | 163.45 | 77.49 |
12 mA | 3.71×105 | 2.10×10-8 | -0.281 | 168.05 | 94.258 |
15 mA | 4.94×105 | 6.54×10-7 | -0.310 | 227.2 | 110.2 |
20 mA | 8.77×105 | 8.21×10-7 | -0.325 | 194.71 | 84.54 |
Ingredients | Concentration (g/L) |
NiSO4•6H2O | 120 |
ZnSO4•7H2O | 180 |
Additive 1 | 160 |
Additive 2 | 60 |
NH4OH (28%) | ~200 mL |
pH | 9.3 - 9.5 |
Temperature | RT |
**PQ® Sodium Silicates, PQ Corporation, Malvern, PA
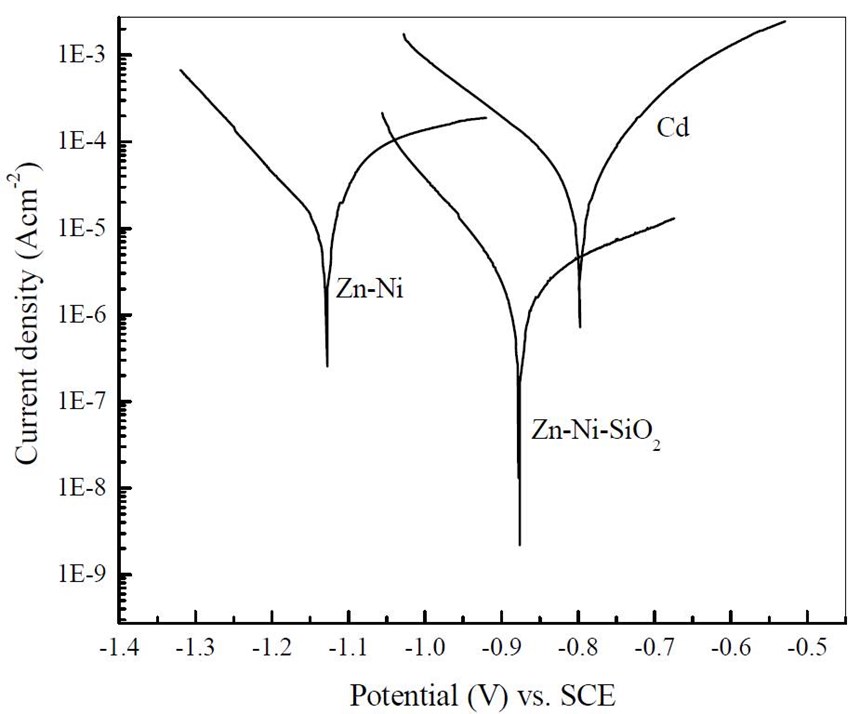
Figure 12 shows the Tafel polarization curves of cadmium, alkaline Zn-Ni and Zn-Ni-SiO2 samples in a 5% NaCl solution. The results obtained by Tafel extrapolation method, as well as the corrosion current and deposit hardness are summarized in Table 6. The corrosion potential of cadmium, Zn-Ni and Zn-Ni-SiO2 are -0.806, -1.127 and -0.822 VSCE, respectively. It should be noted here that the corrosion potential of Zn-Ni after silica passivation has been shifted to a more positive value and is close to that of cadmium coatings, thereby reducing the driving force for rapid corrosion. Also, the corrosion current of the base Zn-Ni (Icorr = 2.17×10-5) is reduced by two orders of magnitude after silica passivation (Icorr = 3.36×10-7). These results indicated that the corrosion resistance of Zn-Ni coatings is greatly improved by silica passivation processes.
Table 6 - Corrosion potential, corrosion current density and Vickers hardness of different samples.
Corrosion potential, ECorr (VSCE) | Corrosion current density, ICorr(A/cm2) | Vickers hardness (HV100) | |
Cd | -0.806 | 3.28×10-6 | 55.92 |
Zn-Ni | -1.127 | 2.17×10-5 | 325.53 |
Zn-Ni-SiO2 | -0.822 | 3.66×10-7 | 315. |
Conclusion
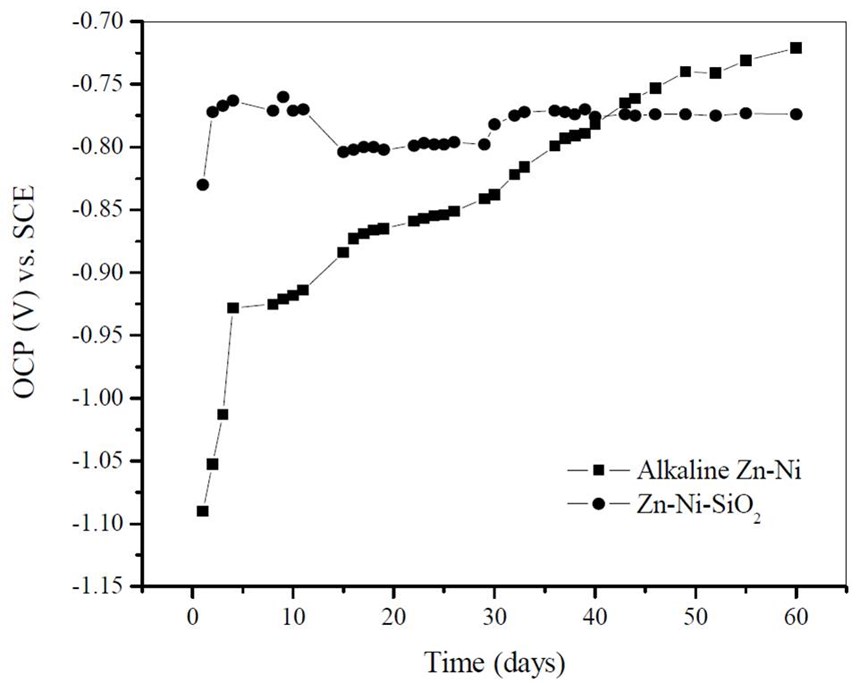
An environmentally-benign silica passivation process was developed for alkaline Zn-Ni deposits. It was shown that a thin silicate layer can be deposited on the Zn-Ni by the in-house developed surface modification procedure followed by immersion in sodium silicate solution. Corrosion studies in 5% NaCl indicated enhanced corrosion protection when compared to the bare Zn-Ni coatings.
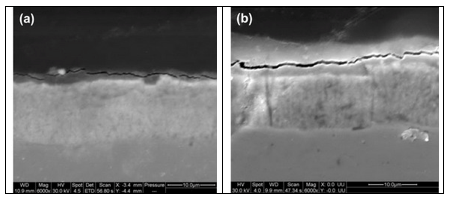
The cross-sectional analysis of the Zn-Ni-SiO2 samples after the 3rd and 7th week of testing is shown in Fig. 15. As shown in the figure, the underlying Zn-Ni deposit is unaffected for the entire exposure period. ASTM B 117 results confirmed that the silica passivation is an effective method to increase the life of the Zn-Ni coatings.
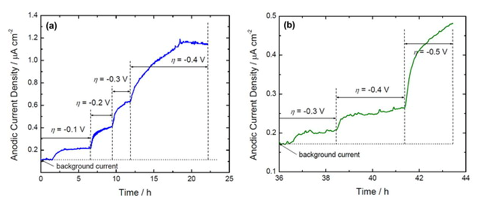
An environmentally benign silica passivation process was developed for alkaline Zn-Ni deposits. ASTM B117 salt spray test results indicated red rust formation after 40 days of exposure for the unscribed Zn-Ni coated sample and no red rust was seen through the scribe after 60 days of exposure in the case of Zn-Ni-SiO2 coated sample. The ASTM B 117 salt spray test confirmed that the silica passivation improved the corrosion properties of Zn-Ni deposits. Hydrogen permeation studies of Zn-Ni-SiO2 showed lower hydrogen permeation current when compared to the bare alkaline Zn-Ni coating at all the applied cathodic overpotentials.
RELATED CONTENT
-
Andrews Powder Coating Earns Space Flight Qualification
The first-tier aerospace powder coating supplier recently had its MIL-SPEC powder coatings qualified for coating power supplies for a classified military satellite program.
-
Evaluation of Hexavalent Chromium Free Bond Primers for Aerospace and Defense Applications
Three hex chrome free bond primers demonstrated that they can surpass the 960 hour threshold before exhibiting panel corrosion away from the scribe.
-
Painting a Boeing 737: See The Video
The cost is between $100,000 to $200,000 to prep and paint each plane, depending on which colors are chosen, and how many colors.