Electrodeposition of Ni-Fe-Mo-W Alloys - 13th Quarter Report
13th Quarterly Report - AESF Research Project #R-117. This NASF-AESF Foundation research project report covers the 13th quarter of project work (January-March 2016).
#nasf
by
Yujia Zhang and E.J. Podlaha-Murphy*
Featured Content
Northeastern University
Boston, Massachusetts, USA
Editor’s Note: This NASF-AESF Foundation research project report covers the 13th quarter of project work (January-March 2016). Progress on the previous quarters has been published in summary in the NASF Report in Products Finishing and in full at www.pfonline.com.** A printable PDF version is available by clicking HERE.
Beginning in January 2013, the NASF, through the AESF Foundation Research Board, funded a three-year project on alloy plating at Northeastern University, in Boston, under the direction of Dr. Elizabeth Podlaha-Murphy, with emphasis on nickel-molybdenum-tungsten deposits. In 2016, a one-year extension was granted to examine the influence of oxide particulates codeposited with different combinations of Ni, Mo and W alloys on the resulting deposit composition in the generation of novel alloy composites. What follows is the first report on this new work.
Electrodeposition of Ni-W-TiO2 composites
Metal matrix composite coatings combine a second phase particle into a metal, and can address the need for advanced materials with tailored properties. There are many reports of different particle types electrodeposited with metals, with several good reviews available.1,2 Generally, the particle is dispersed in an electrolyte, commonly by mechanical means through stirring, and captured into a growing film as the metal ion is reduced at the electrode surface. In particular, electrodeposited Ni-W alloys with titania particles have been previously reported with an emphasis on how the particle affects one of the deposit properties and its structure. Kumar, et al.3 examined the corrosion resistance and hardness of nickel-rich Ni-W deposits with small amounts of titanium. The hardness was improved with the inclusion of particles and the corrosion resistance was enhanced, particularly for pulsed deposition of the composite. Goldasteh and Rastegari,4 also reported improved hardness for a similar Ni-W-TiO2 composite compared to a Ni-W alloy. They used nano-sized titania particles leading to grain refining. Only when their composite was pulse deposited was there an enhancement of corrosion resistance in a 0.5M NaCl electrolyte. Other oxide particle types have been demonstrated with Ni-W alloy matrices as well, including nano-scaled alumina and zirconia. Yari and Dehghanian5 found that the alumina particle did not change the surface morphology, phase or texture of the Ni-W, but decreased the tungsten content and the rate of deposition. Beltowska-Lehman, et al.6 reported an enhancement of hardness with Ni-W-ZrO2 compared to similarly deposited Ni-W. They utilized ultrasonic agitation that can help to disperse the particles in the electrolyte and found that it had a positive effect on the amount of particle in the deposit.
Typically, Ni-W is electrodeposited from ammonium-containing electrolytes, and results in nickel-rich alloys, as in the cited examples above. Without ammonium ions in the electrolyte, the amount of tungsten in an electrodeposited film has been shown to be higher in Ni-W alloys, although with a loss in current efficiency.7 Presented here is an examination of adding titania particles to an ammonia-free Ni-W electrolyte, resulting in high amounts of tungsten in the deposit. Our goal is in determining if the particle changes the rates of deposition (i.e., partial current densities), hence composition and current efficiency, and if the inclusion of particles affects the morphology.
Experimental
A citrate-boric acid electrolyte was used in this study and contained 0.1M nickel sulfate, 0.15M sodium tungstate and 0.285M trisodium citrate, 1.0M boric acid, at a pH of 8 adjusted with sodium hydroxide, with and without 12.5 g/L micron-size titanium dioxide (particle diameters less than 44 microns, -325 mesh, Acros Organics). The determination of polarization curves and actual deposition were carried out at room temperature on copper covered brass cylinder electrodes, having a diameter of 0.6 cm. A copper tape, (3M Copper Conducting Tape, SPI supplies), typically used for SEM analysis, was used to cover the electrode so that the film could be readily removed for further analysis and the cylinder current collector re-used. The polarization data was scanned from the open circuit potential to a large enough overpotential to achieve a current density that is reached in a rotating Hull cell configuration. During the polarization scan a three-electrode cell configuration was used with a platinum mesh anode and a saturated calomel reference electrode (SCE). The curves were corrected for ohmic drop with impedance spectroscopy. The rotating Hull cell, shown in Fig. 1, used the same diameter rod, with a length of 8 cm, and an average current density of 50 mA/cm2. A plastic cylinder placed between the anode and the cathode, create a current distribution. The plot in Fig. 1 provides a way to estimate the current distribution. The calculated values assume a primary current distribution. The rotation rate was 500 rpm. A Solartron 1287A potentiostat/galvanostat current generator was used for both linear sweep voltammetry and deposition.
The composition and thickness were measured using x-ray fluorescence. The deposit surface morphology was inspected with scanning electron microscopy (SEM) obtained using a Hitachi 4800 at different magnifications.
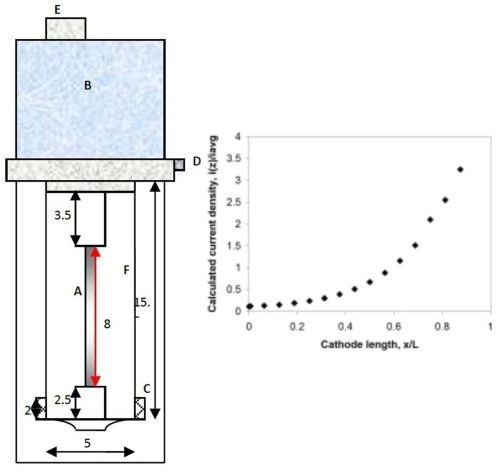
Figure 1 - Schematic diagram of a rotating Hull cell (measurements in cm) with the current distribution expected when reactions are facile (primary c.d.): (A) working electrode, (B/E) motor, (C) concentric anode, (D) electrical contact and (F) plastic shield; dimensions in cm. Design by Madore and Landolt.8
Results
The conditions selected for this study are intended to result in high tungsten deposit composition. Figure 2 shows the polarization curves of Ni-W with and without titania particles added to the electrolyte using a rotating cylinder working electrode, not in a Hull configuration, but with uniform current distribution. The scan rate was 2 mV/sec and the electrode rotation rate was 500 rpm. Both polarization curves are very similar. The noise generated at potentials more negative than -1.2 VSCE is attributed to the large water reduction side reaction.
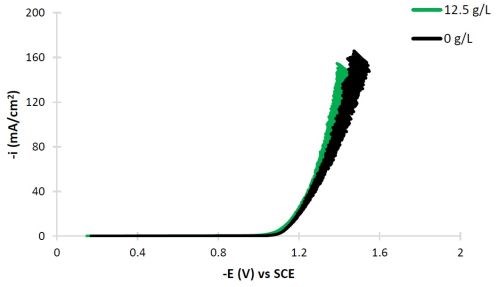
Figure 2 - Polarization curves of the Ni-W, citrate-boric acid electrolyte at 500 rpm, room temperature with and without titania particles.
The polarization data does not clearly reflect changes made in the deposit with the addition of particles, as the side reaction is dominant in this electrolyte. Thus, deposits were studied in a rotating Hull cell to deposit the alloy and composite over a large range of current density at the same rotation rate, hence mixing environment. In the rotating Hull cell configuration, the deposit morphology and composition was characterized. Figure 3 compares two samples without and with the addition of particles in the electrolyte. The high current density end (~150 mA/cm2) is at the bottom of the electrode and the lowest (~5 mA/cm2) is at the top. A significant change in the deposit morphology was observed with the addition of particles in the electrolyte (Fig. 2). With particles added, the deposit became duller and rougher. SEM studies were undertaken in the center region of the rotating Hull cell, within the red rectangle shown in Fig. 3.
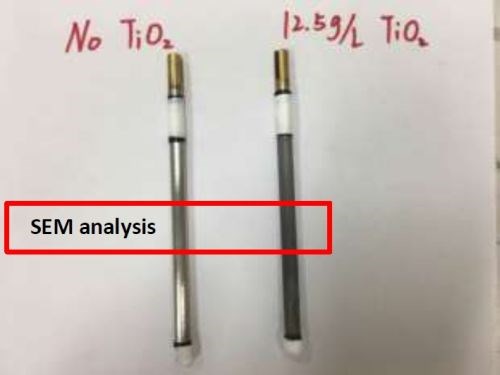
Figure 3 - Ni-W deposit on a rotating Hull cell without particles (L) and with particles (R), SEM images of the region outlined.
Figures 4 and 5 show SEM images of a comparable Ni-W deposit without particles in the electrolyte, Fig. 4(a,b), and with them present, Fig. 5 (a,b), at different magnification, from the center region of the rotating Hull cell in Fig. 3. Micro-size cracks are evident in both cases, but the surface morphology changed significantly with the addition of particles in the electrolyte, becoming more nodular. Both Figs. 4(b), Ni-W alloy without the titania particles, and 5(b), with particles are at the same magnification, 20,000×, illustrating that the deposit growth is more three dimensional when particles are present, with nodules growing on the top of other nodules. Also of interest is the observation that no titania particles larger than one micron were observed on the composite surface, although XRF and SEM-EDS analysis did confirm the presence of titanium.
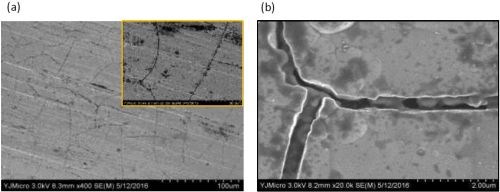
Figure 4 - SEM images of Ni-W alloy when no titania particles are present in the electrolyte, at (a) low and (b) high magnification.
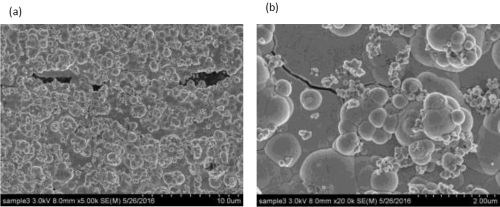
Figure 5 - SEM images of the Ni-W alloy when 12.5 g/L titania particles are present in the electrolyte at (a) low and (b) high magnification.
(a)
(b)
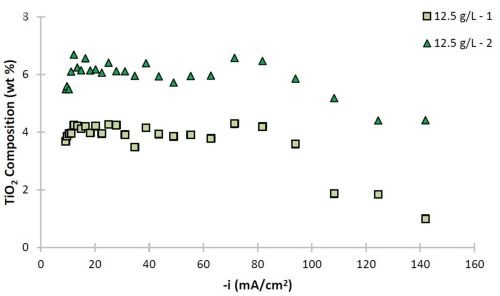
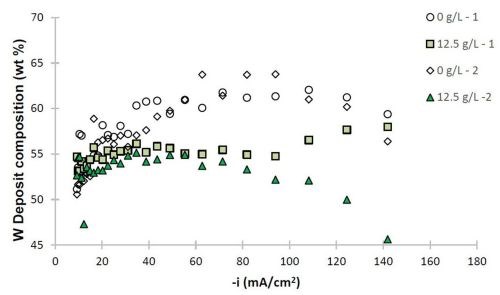
Figure 6 - Ni-W and Ni-W-TiO2 deposit composition (wt%): (a) W and (b) TiO2.
The deposit composition along the rotating Hull cell length is presented in Fig. 6. The x-axis represents an estimated current density at each electrode position assuming a primary current distribution (see scale in Fig. 1). Duplicate samples are shown to assess the variability in composition. In the absence of particles, shown as open circle and diamond data points, the tungsten content varied between 50 and 63 wt% along the electrode. The composition was slightly affected by the presence of particles, with decreasing tungsten at cathodic current densities significantly larger than 40 mA/cm2. The amount of titania particles codeposited was roughly constant over a large current density range, and decreased only at very high current density values >100 mA/cm2. In the region where there is a small decrease in tungsten content, there is also a large side reaction rate. The two data sets presented with 12.5 g/L of titania particles in the electrolyte show that the resulting amount of particles in the deposit varied by about 2 wt%, with the largest variability occurring when the hydrogen evolution side reaction was largest, at current density values >100 mA/cm2. Also in this range, the tungsten content in the Ni-W alloy showed the greatest variation between the two samples. Thus, the hydrogen evolution reaction may be contributing significantly to the transport of the particles in a stochastic way.
The morphological changes shown in Figs. 4 and 5 correspond to similar changes in deposit composition. Without particles, the tungsten content in the Ni-W alloy is ~60 wt%, while without particles, it is lower, at ~55 wt% with 6 wt% titania particles in the Ni-W-TiO2 composite.
(a)
(b)
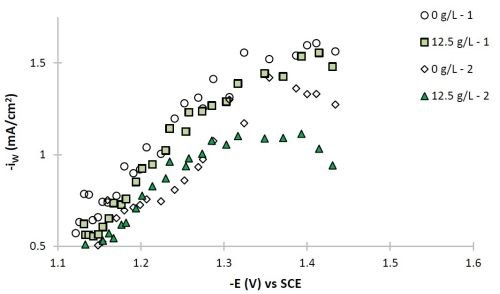
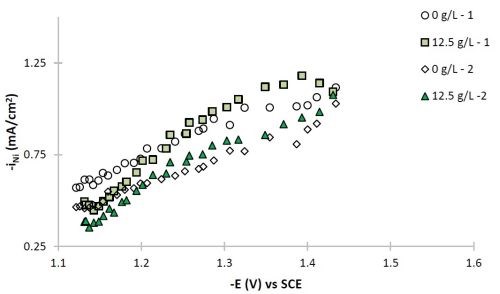
Figure 7 - Partial current densities of (a) nickel and (b) tungsten.
The polarization curves of Fig. 2, allow for a correlation between the estimated current density and the working electrode potential. Using the thickness measurement provided by XRF, and Faraday’s law, the partial current densities can be determined. Figure 7 shows the partial current densities of (a) nickel and (b) tungsten, with and without particles for the two sets of data replicating the particle-free electrolyte and the electrolyte with particles. The current density scale has now been replaced with the working electrode potential. At low total current densities, or more noble potentials, the partial current densities of both nickel and tungsten increase with potential and are hence kinetically controlled. There is no significant change of the metal reduction partial current densities with or without particles. At higher total current densities, there is more variability in the data and the tungsten deposition rate starts to be influenced by mass transport. It is in this region where the tungsten content in the deposit decreases.
The current efficiency can also be estimated with the use of the primary current density assumption. The current efficiency is relatively low for this particular system (Fig. 8), as expected for high tungsten content in the Ni-W deposit. The particle addition does not appreciably change the current efficiency.
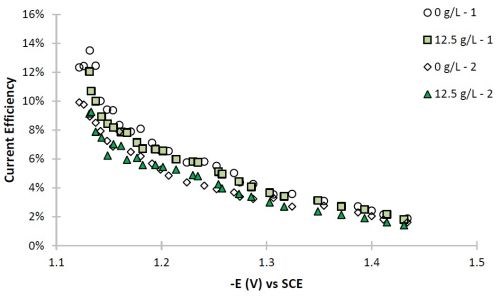
Figure 8 - Partial current densities of (a) nickel and (b) tungsten.
Conclusions
Titania particles (325 mesh) were added to a Ni-W electrolyte to electrodeposit Ni-W-TiO2 composites with high tungsten wt %. The particles added to the electrolyte did not significantly alter the current efficiency or composition; however, it did drastically change the morphology and thus growth of the deposit.
Further work is underway to examine the influence of particle concentration on Ni-W morphology, and exploring the electrodeposition of Ni-W-Mo-TiO2.
References
1. A. Hovestad and L.J.J. Janssen, “Electroplating of Metal Matrix Composites by Codeposition of Suspended Particles,” in Modern Aspects of Electrochemistry, No. 38, B.E. Conway (Ed.), Kluwer Academic/Plenum Publishers, New York, 2005.
2. C.T.J. Low, R.G.A. Wills and F.C. Walsh, Surf. Coat. Technol., 201 (1-2), 371-383 (2006).
3. K.A. Kumar, G.P. Kalaignan and V.S. Muralidharan, Ceram. Int., 39 (3), 2827-2834 (2013).
4. H. Goldasteh and S. Rastegari, Surf. Coat. Technol., 259 (Part C), 393-400 (2014).
5. S. Yari, and C. Dehghanian, Ceram. Int., 39 (7), 7759-7766 (2013).
6. E. Beltowska-Lehman, et al., Mater. Design, 80, 1-11 (2015).
7. O. Younes and E. Gileadi, J. Electrochem. Soc., 149 (2), C100-C111 (2002)
8. C. Madore & D. Landolt, Plating & Surface Finishing, 80 (11) 73-78 (1993); also republished as: http://www.pfonline.com/cdn/cms/1501_Printable_Version.pdf.
Footnotes
*Corresponding author:
Prof. E.J. Podlaha-Murphy
Professor of Chemical Engineering
Northeastern University
Boston, Massachusetts 02115
Phone: (617) 373-3796
E-mail: e.podlaha-murphy@neu.edu
**Quarter 1 (January-March 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (1), 11-17 (October 2013); http://short.pfonline.com/NASF13Oct2.
Quarter 2 (April-June 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (2), 18-27 (November 2013); http://short.pfonline.com/NASF13Nov2.
Quarter 3 (July-September 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (4), 11-16 (January 2014); http://short.pfonline.com/NASF14Jan2.
Quarters 4-6 (October 2013 - June 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (2), 1-14 (November 2014); http://short.pfonline.com/NASF14Nov1.
Quarter 7 (July - September 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (7), 1-9 (April 2015); http://short.pfonline.com/NASF15Apr1.
Quarter 8 (October - December 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (10), 1-8 (July 2015); http://short.pfonline.com/NASF15Jul1.
Quarter 10 (April - June 2015): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 80 (7), 1-8 (April 2016); http://short.pfonline.com/NASF16Apr1.
Quarters 11-12 (July - December 2015): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 80 (12), 1-10 (September 2016); http://short.pfonline.com/NASF16Sep1.
About the authors:


RELATED CONTENT
-
Development of a Sustainability Metrics System and a Technical Solution Method for Sustainable Metal Finishing: AESF Research Project #R-121, 6th Quarterly Report
The NASF Research Board has funded a research grant at Wayne State University on sustainability in the surface finishing industry, under the direction of Professor Yinlun Huang. The objective of the work is to create a surface-finishing-specific sustainability metrics system to measure economic, environmental and social sustainability. In this report, a benchmarking study of five plants was undertaken to illustrate how the sustainability assessment works.
-
Mechanical Properties of Electroformed Metals
In 1996, the AESF held its highly regarded electroforming course, prepared by Ron Parkinson for presentation by The Nickel Development Institute (NiDI) and the AESF. What follows is a slightly modified excerpt, specifically on the mechanical properties of electroformed metals. Much of this information has withstood the test of time, and gives a perspective of this technology at the turn-of-the-century.
-
A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .