Electrodeposition of Ni-Fe-Mo-W Alloys - Part 9
Ninth Quarterly Report - AESF Research Project #R-117. This NASF-AESF Foundation research project report covers the ninth quarter of project work (January-March 2015). In this period, Graduate student Avinash Kola has continued work on the influence of deposition conditions on the properties of the Ni-W alloys.
#research #nasf #surfin
by
A. Kola and Prof. E.J. Podlaha-Murphy,*
Featured Content
Northeastern University
Boston, Massachusetts, USA
Editor’s Note: This NASF-AESF Foundation research project report covers the ninth quarter of project work (January-March 2015). Progress on the previous quarters has been published in summary in the NASF Report in Products Finishing and in full at www.pfonline.com.** A printable PDF version is available by clicking HERE.
Introduction
The project, initiated in January 2013, addresses the induced codeposition of molybdenum and tungsten alloys with nickel and iron having a focus on developing a toolbox of plating conditions to deposit different combinations of Ni, Fe, Mo and W. Through NASF-AESF Foundation funding, several students, both graduate and undergraduate, have gained experience in surface finishing research. This paper covers progress made during the ninth quarter. In this period, Graduate student Avinash Kola has continued work on the influence of deposition conditions on the properties of the Ni-W alloys.
In our last report interesting electrodeposition behavior was observed with the additive 2-butyne-1,4-diol (BD) in a Ni-W electrolyte at low pH. This additive has been reported in the literature with Ni-W codeposition for the purpose of creating a more corrosion resistant deposit. In the 8th quarter, graduate student Avinash Kola examined parameters that influenced Ni-W deposition, with a notable change of composition reported with the addition of BD. The more BD added to the electrolyte, the higher the nickel content in the resulting deposit. The partial current density evaluation found that it was not the rate of the tungstate reduction that was altered but rather an increase in the nickel rate. It was an unexpected result since it has been documented in the literature that BD should cause nickel to be inhibited.1-3 To examine this behavior further, nickel was electrodeposited under the same conditions as Ni-W in a low pH citrate electrolyte in order to compare with our previous findings. In addition, we have started to examine the behavior at a higher pH in an effort to improve the current efficiency.
Experimental
In this report all depositions were carried out in the traditional Hull cell with air agitation. The electrolyte used in this study contained 0.1M nickel sulfate, 0.15M sodium tungstate and 0.285M trisodium citrate at a pH of 2 and at room temperature. The BD concentration was varied from 0 to 5 mM. The average applied current density was 50 mA/cm2 on a copper plate in a Hull cell configuration for a duration of 30 min and at an agitation rate of 3 L/min (air). Polarization data was obtained by placing the electrodes parallel to each other, at a scan rate of 10 mV/sec, and with a Ag/AgCl reference electrode. The curves were corrected for ohmic drop with impedance spectroscopy. Composition and thickness were measured using x-ray fluorescence.
Ni electrodeposition in a low pH electrolyte (to compare with Ni-W)
In our previous report, and resulting paper publication in Products Finishing (http://short.pfonline.com/NASF15Jul1), a polarization curve of Ni-W with different amounts of BD showed a depolarizing effect, which was unexpected. In order to evaluate if this unusual trend in BD addition was due to the nature of the low pH electrolyte or the presence of tungstate, a nickel-only electrolyte was examined. Figure 1 shows the polarization behavior when elemental nickel is electrodeposited with different amounts of BD. Consistent with literature reports, there is a small inhibition of the polarization behavior seen at 5 mM BD, and with all BD additions at the low current density region. At higher current densities, there is a smaller difference. However, in this region considerable hydrogen evolution occurs. Figure 2 compares the different polarization curves of the elemental nickel and Ni-W alloy electrolytes at (a) no additive (b) 1 mM BD, (c) 2.5 mM BD and (d) 5 mM BD. The most striking observation is that, without the additive, the nickel-only deposition current density is larger than that of the Ni-W case at a given current density. However, once BD is added to the electrolyte the total current density in Ni-W electrolytes is larger than that for the elemental nickel. The larger the amount of BD, Figure 2 (b,c,d), the larger the difference between the polarization curves of nickel and Ni-W.
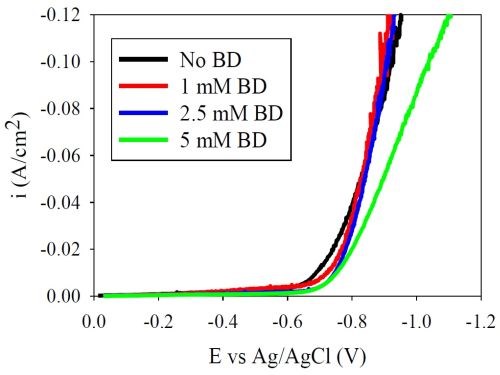
Figure 1 - Nickel-only electrolyte with variable amounts of BD (for review from the previous quarter’s work).
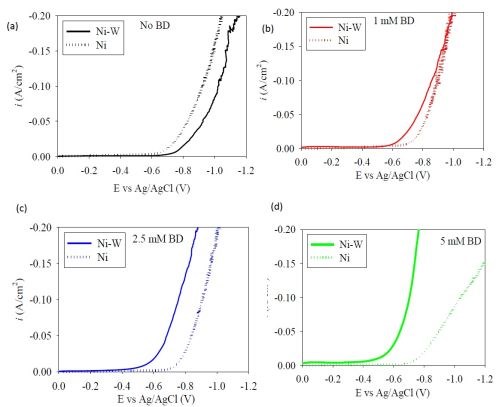
Figure 2 - Polarization curves of Ni-only and Ni-W electrolytes with variable amounts of BD.
To address the nature of this difference, the partial current densities were examined. In our last report, we found that the nickel partial current densities during alloy deposition were indeed affected by BD, but in an unusual way: the nickel partial current densities were shifted to more positive potentials. The tungsten partial current density did not change significantly with BD. When tungstate is not present, the change in the polarization curve could be accounted for by a decrease in the nickel partial current density, or in other words, a shift in potential to the negative direction. To see if this is indeed true the partial current densities were estimated from Hull cell experiments.
Figure 3 shows photographs of the Hull cell deposits (a) without BD, and with the following concentrations of BD: (b) 0.1 mM, (c) 2.5 mM and (d) 5 mM. The chosen applied current density captures the low current density region where only the side reaction occurs, a mid-range region where the nickel appears powdery-black, and then a higher current density region where the deposit is metallic. Air agitation was used in the Hull cell and the photographs show that the deposit reflects streaks from the agitation only when BD is present, due to the indirect action of BD on the metal deposition by mass transport. Note the absence of streaks when BD is not present. In addition, an increase in BD transitions the deposit to a more brilliant surface aspect, as expected, since BD is a representative class II brightening agent.4
The partial current densities were determined from the deposit thicknesses that were estimated from the XRF, scanning locally across the electrode surface and assuming that the density of nickel was its bulk value. The thickness results in Fig. 4 have correlated the position along the electrode with an estimated current density, assuming a primary current distribution. The thickness increases with the estimated applied current density for all BD electrolytes and there is little difference at the low current density region. At higher current density, there is a substantial change in thickness with BD, with a thinner deposit resulting with additions of BD in the electrolyte. Using Faraday’s law, the partial current density shown in Fig. 5 clearly shows that the result of the decreased thickness is not due to a larger side reaction, but rather due to an inhibition of the nickel reaction rate with increasing BD. Figure 5 uses the polarization curves in Figs. 1 and 2 to transform the current density to an estimated applied potential, more useful for comparing the effect of the driving force of the reaction. In our last report, the nickel partial current density when codeposited with tungsten did change substantially with BD, increasing with BD concentration, but here, when nickel is electrodeposited alone, the nickel partial current density again changes with BD electrolyte concentration but in a different manner. The BD lowers, or inhibits, the nickel reduction reaction. Thus, the differences in polarization curves for the Ni-W and nickel electrolytes with BD at pH 2 reflect the action of the nickel rate. It is inhibited with BD when tungsten is not present, but enhanced when tungsten is present. The de-polarizing effect observed in the polarization curves with BD in our last report, in a Ni-W electrolyte at pH 2, is thus attributed to the presence of tungsten through an indirect action on the nickel reaction rate.
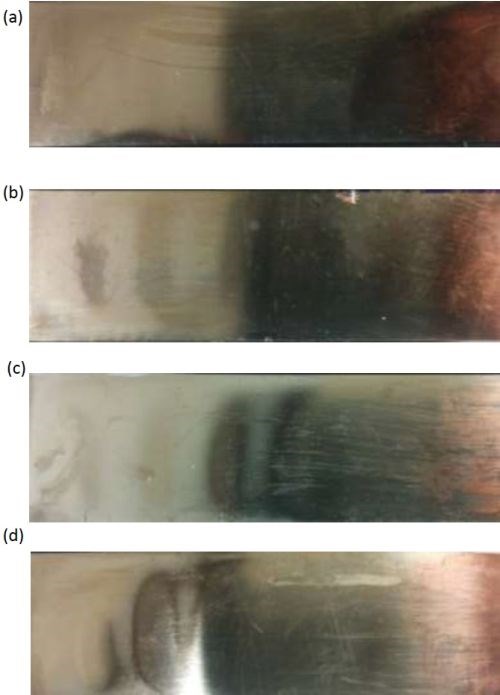
Figure 3 - Hull cell nickel deposits with varying amounts of BD in the electrolyte (a) 0, (b) 1 mM, (c) 2.5 mM and (d) 5 mM.
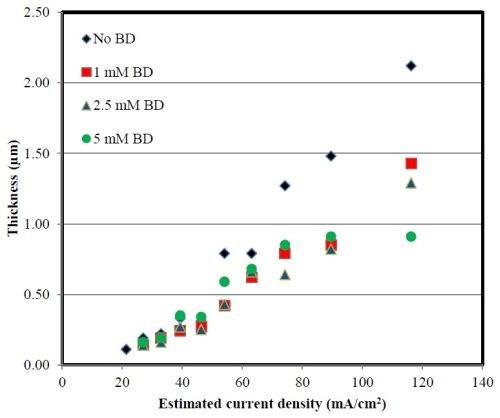
Figure 4 - Deposit thickness across the Hull cell correlated with an estimated current density for different amounts of BD in the electrolyte.
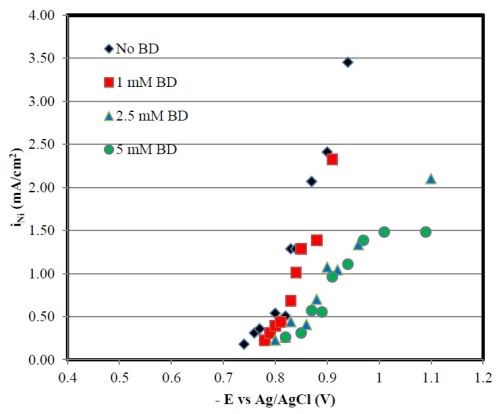
Figure 5 - Nickel partial current density for different amounts of BD in the electrolyte.
Ni-W electrodeposition with BD in a high pH citrate electrolyte
Typically, Ni-W is electrodeposited at high pH with an appropriate complexing agent for nickel ions to avoid its precipitation. Tungstate and tungstate-citrate species are soluble at both low and high pH. A higher pH can also help to increase the current efficiency.
Figure 6 shows polarization curves of the Ni-W electrolyte at pH 8 with different amounts of BD. Again there is a depolarizing effect, a shift in the curves in a positive direction, with the addition of BD. Recall that in the last report, at pH 2, a similar behavior was observed for the same Ni-W polarization.
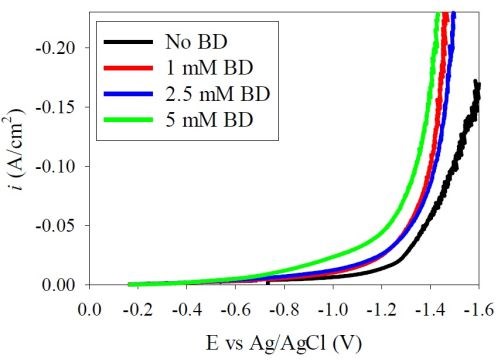
Figure 6 - Polarization curves at pH 8 in the Ni-W electrolyte.
Figure 7 reports the composition behavior at pH 8 along the Hull cell at the estimated current densities. All alloys are nickel-rich. An increase in BD tends to increase the amount of nickel in the low-to-mid current density range, similar to the pH 2 case. At very high applied current densities, there is less difference in composition.
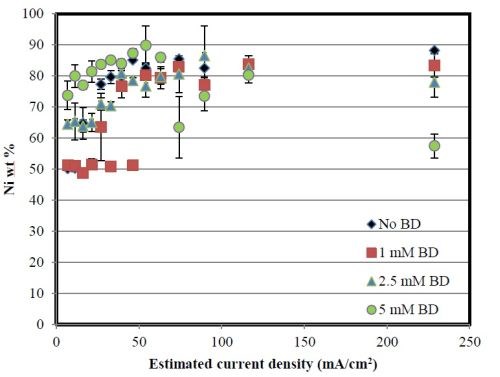
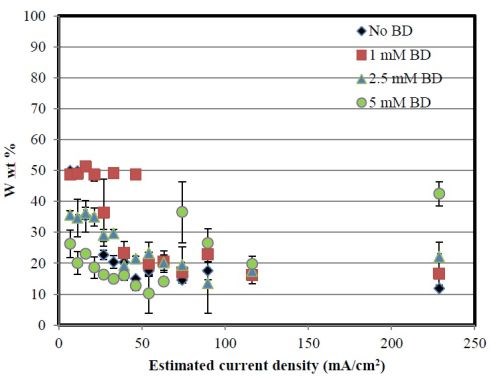
Figure 7 - Deposit composition of nickel and tungsten at the estimated applied current densities, pH 8.
The deposit thickness is provided in Fig. 8. An addition of 1 mM BD produces no substantial change on the deposit thickness, but at 2.5 and 5 mM BD, there is a significant decrease in the deposit thickness. In general, the deposit thickness for the time of deposition is much lower than even pH 2 when BD is added to the electrolyte. In all cases, the deposits were shiny and reflective (Fig. 9) and also display the streak-lines from the air agitation.
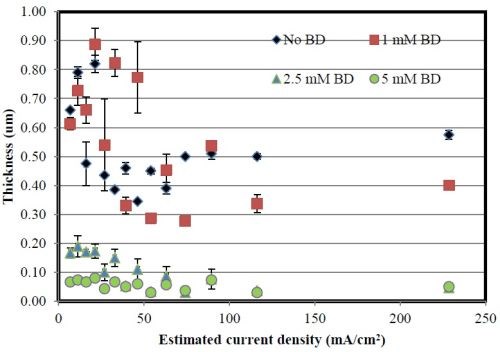
Figure 8 - Deposit thickness of Ni-W deposits at pH 8.
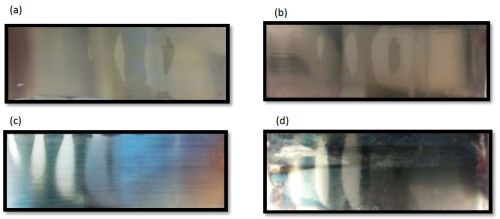
Figure 9 - Ni-W deposit along a Hull cell with (a) no BD, (b) 1 mM BD, (c) 2.5 mM BD and (c) 5 mM BD, at pH 8.
Inspection of the partial current densities (Fig. 10) shows a substantial decrease in both the nickel and tungsten partial current densities with BD. Unlike the pH 2 case, the increase in the nickel deposit content is due to the larger decrease in the tungsten partial current density as compared to the nickel partial current density. This behavior is consistent with the reported action of BD on nickel reduction, leading to a decrease of the nickel partial current density with BD concentration in the electrolyte.
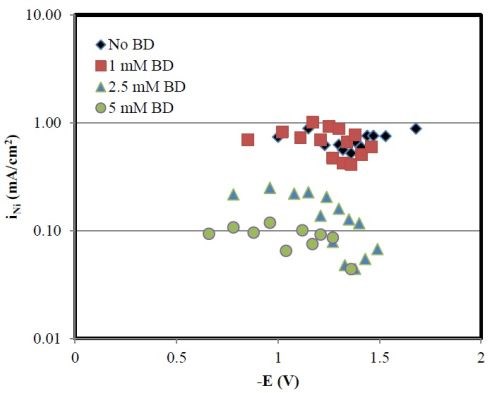
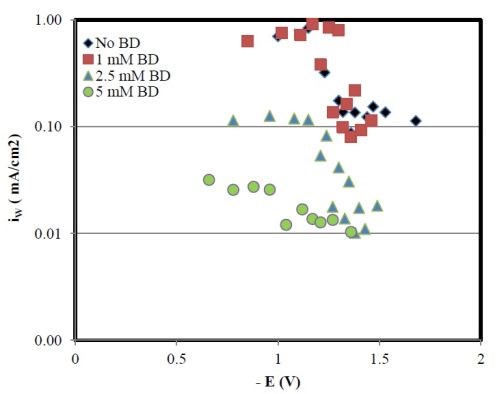
Figure 10 - Partial current densities of nickel and tungsten with various amounts of BD, at pH 8.
If both the metal partial current densities decrease at pH 8 with BD then why do the polarization curves (Fig. 6) appear larger with an increase in BD? The increase in the total current density then must be due to an enhanced side reaction.
Comparing results from Report #8 and this Report #9 for Ni-W deposition in pH 2 and pH 8, respectively, the partial current density behavior is very different with the change in the BD additive. This point highlights the need to be wary not to over-generalize additive behaviors on metal deposition rates. We suspect that the different behaviors may be fundamentally based on the different species that are at equilibrium in the bulk solution at these two different pH values. Table 1 summarizes some of the key equilibria and metal-citrate species that can be generated by the citrate ligand (Cit = C6O7H5), assuming that the nickel and tungstate salts completely dissociate to form Ni+2 and WO4-2. It is not a comprehensive list of all the species, and has not included the polytungstate species expected at low pH. Together with Table 1 and mass balances of the nickel, tungsten and citrate species, Fig. 11 is generated, providing a better guide as to what species dominate at the two different pH values of study. At pH 2 the nickel species is primarily the NiHCit2-3 species. At pH 8 the unprotonated nickel species dominate, NiCit2-4 and NiCit-. The tungsten species change in a similar way. At pH 2 the protonated tungsten-citrate species are present WO4CitH3-2 and at pH 8 there is free tungstate species, WO4-2, and fewer protonated tungstate-citrate species develop. Thus, the changes in the effect of BD on the nickel rate may be due to both the change of the nickel species as well as from the indirect effect of the change in the tungsten species with pH.
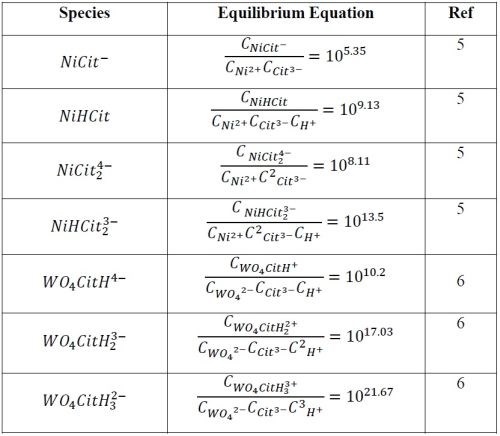
Table 1 - A selection of the most significant Ni- and W-citrate species and equilibria.
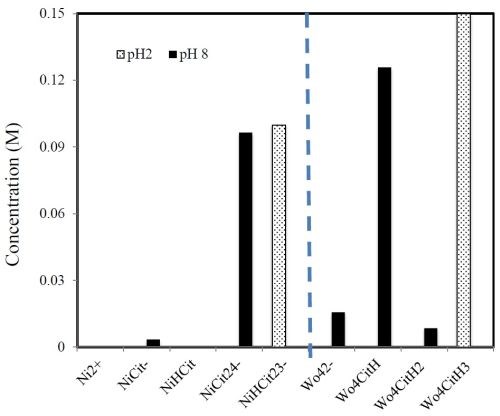
Figure 11 - A summary of some of the possible species present in the pH 2 and 8 electrolytes.
Conclusions
In our previous report, the effect of BD on Ni-W in a low pH environment was examined. The increasing concentration of BD affected the nickel deposition rate, shifting it to more positive potentials. This behavior is in contrast to nickel deposition as an elemental deposit that exhibited a decrease in its partial current density with BD added to the electrolyte. No shift in tungsten deposition rate was observed. However, a different trend was observed when a high pH electrolyte was examined. The nickel reaction rate decreases with increasing BD at pH 8. Also, unlike the low pH case, the tungsten deposition rate decreases dramatically with increasing concentration of BD. This behavior leads to a lowering of the current efficiency, and more nickel in the deposit. The difference in behavior observed between the low and high pH electrolytes, is attributed to the different species present at different pH values. Different Ni-Cit and WO4–Cit complexes can possess different reduction rates, via mechanisms that can be sensitive to adsorbed intermediates, resulting in the different observed behavior.
Future work
Future work will look at the effect of boric acid and temperature on the behavior of BD towards Ni-W deposition. It is also planned to characterize the resulting structure with XRD to complete the project.
Presentations
Poster presentation
Part of this work was presented in a poster presentation in the Department of Chemical Engineering, Northeastern University for visiting potential graduate students, at the Graduate Student Open House Event, March 2015. Support by the AESF Foundation was acknowledged.
Oral presentation
This work was presented at the 2015 NASF Sur/Fin Annual Meeting in Chicago, Illinois.
References
1. R. Srinivasan and G.N.K. Ramesh Bapu, Trans. Inst. Metal Fin., 91 (1), 52 (2013).
2. J.H. Lim, et al., J. Electrochem. Soc., 156 (3), D108 (2009).
3. E.A. Pavlatou, M. Raptakis and N. Spyrellis, Surf. & Coat. Technol., 201 (8), 4571 (2007).
4. H. Brown, Proc. Interfinish, Basel, Switzerland, 1972, p. 114.
5. T.A. Green, A.E. Russell and S. Roy, J. Electrochem. Soc., 145 (3), 875 (1998).
6. J.J Cruywagen, L. Krüger, and E.A. Rohwer, J. Chem. Soc., Dalton Trans., 1991 (7), 1727 (1991).
About the authors:

Avinash Kola is a Ph.D. student in the Department of Chemical Engineering at Northeastern University. He received his M.S in Chemical Engineering at Louisiana Tech University in 2010, and his B.S from Jawaharlal Nehru Technological University, India in 2006.
Footnotes
*Corresponding author:
Prof. E.J. Podlaha-Murphy
Professor of Chemical Engineering
Northeastern University
Boston, Massachusetts 02115
Phone: (617) 373-3796
E-mail: e.podlaha-murphy@neu.edu
**Quarter 1 (January-March 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (1), 11-17 (October 2013); http://short.pfonline.com/NASF13Oct2.
Quarter 2 (April-June 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (2), 18-27 (November 2013); http://short.pfonline.com/NASF13Nov2.
Quarter 3 (July-September 2013): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 78 (4), 11-16 (January 2014); http://short.pfonline.com/NASF14Jan2.
Quarters 4-6 (October 2013 - June 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (2), 1-14 (November 2014); http://short.pfonline.com/NASF14Nov1.
Quarter 7 (July - September 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (7), 1-9 (April 2015); http://short.pfonline.com/NASF15Apr1.
Quarter 8 (October - December 2014): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 79 (10), 1-8 (July 2015); http://short.pfonline.com/NASF15Jul1.
RELATED CONTENT
-
Hybrid Sol-Gel Coatings in Surface Engineering
A look at the use of modified sol-gel polymer films and hybrid system coatings, as well as the methodologies for evaluating the mechanical properties of the coatings.
-
Cyanide Destruction: A New Look at an Age-Old Problem
Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
-
A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .


















