Environmentally Friendly Fastener Finishes
A research study was conducted on eight finishes that are potential replacements for cadmium. Information from the tests is specific to clamp load and corrosion, both cosmetic and galvanic...
"The Perfect Finish" is something the industry has sought for years. It is an elusive concept on which millions of dollars have been spent developing, testing and qualifying possible alternative finishes, but most of these efforts have been futile. Each finish, from phosphate to cadmium, has strengths and weaknesses that must be weighed for each application. Using these considerations, progress can be made toward using the materials that have the closest resemblance to the strengths of cadmium that are required and, in turn, accepting their weaknesses.
This paper describes a research study conducted on eight finishes that are potential replacements for cadmium. Information is specific to fasteners with regards to clamp load and corrosion, both cosmetic and galvanic. The scope was broadened to understand many elements of each finish to give engineers information vital to recommending their use as cadmium substitutes and exposing weaknesses of each finish. One inorganic alternative was found to be a drop-in replacement for cadmium, and another two were found to closely resemble cadmium's performance in all respects except for electrical conductivity.
Featured Content
Because cadmium offers excellent corrosion resistance, consistent torque-tension, bimetallic compatibility and thickness within standard thread tolerances, it has been most engineers' finish of choice for many years. It is still used in many applications that cannot sacrifice any of the qualities that cadmium offers.
Initially, automotive OEMs established a deadline to remove cadmium by 1995. Chrysler enacted testing programs to fill the hole in its fastener finish requirements.1 Chrysler conducted a Design of Experiment (DOE) to qualify alternatives that met strict performance requirements and also adhered to OSHA and EPA regulations. This DOE resulted in the selection of the Dacromet 320® L coating system because it closely resembled cadmium in fastener applications. As a result, Chrysler was compliant with OSHA and EPA regulations prior to the established deadline. Metal Coatings International Inc. (MCII) was involved with this DOE.
Because of the extreme use of its equipment in critical situations, the military continued to use cadmium for many applications. The delay in switching from cadmium-plated hardware proved beneficial because automotive OEMs compiled much information during that time. The military sorted through the data produced by automotive qualifications and selected the coatings that performed well in the predetermined areas, which in-turn resulted in a substantial cost savings.
Three years ago, the Army embarked on a cadmium replacement journey, testing numerous finishes as potential candidates.2 Although no "perfect finish" was found, this testing resulted in the qualification of a solvent-based coating that closely resembled cadmium with regards to corrosion protection, bi-metallic compatibility and clamp load retention. The weaknesses exposed were lack of conductivity, high coating thickness and the dependence on a supplemental lubricant to meet Army torque charts. Another attribute that must be considered is that this coating was solvent-based and therefore high in volatile organic compounds (VOC). Due to the VOC content, application facilities required expensive air treatment equipment to reduce pollution that otherwise would have escaped into the environment.
|
TABLE I-Torque-Tension
|
||||||||
|
(Completed before testing on separate parts)
|
||||||||
|
K-Factor
|
Avg. Clampload (lb)
|
Load Range-PT (lb)
|
Load Range-FR (lb)
|
|||||
| Finish |
PT
|
FR
|
PT
|
FR
|
High
|
Low
|
High
|
Low
|
| Dacromet 500®B |
0.190
|
0.174
|
11,384
|
12,404
|
13,527
|
9,240
|
14,023
|
10,785
|
| Cadmium + Yellow |
Not Tested
|
0.214
|
10,074
|
11,937
|
8,211
|
|||
| Cadmium + Wax |
0.131
|
0.116
|
16,483
|
18,701
|
19,844
|
13,123
|
22,554
|
14,848
|
| Dacromet 320® L |
0.132
|
0.131
|
16,328
|
16,442
|
18,015
|
14,641
|
18,204
|
14,680
|
| Geomet® L |
0.120
|
0.124
|
17,999
|
17,445
|
19,449
|
16,549
|
18,505
|
16,385
|
| Coating 6 |
0.157
|
0.156
|
13,720
|
13,882
|
15,412
|
12,028
|
15,559
|
12,205
|
| Dacromet 320® XL |
0.087
|
0.095
|
20,875*
|
18,989
|
22,850
|
18,656
|
20,539
|
17,440
|
| Geomet® XL |
0.086
|
0.096
|
20,875*
|
18,718
|
21,919
|
19,831
|
19,368
|
18,068
|
| Zinc + Yellow |
Not Tested
|
0.175
|
Not Tested
|
12,355
|
Not Tested
|
Not Tested
|
15,347
|
9,363
|
| Tin Zinc |
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not
Tested |
Not
Tested |
| Coating 7 |
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not Tested
|
Not
Tested |
Not
Tested |
|
* @ 75 ft-lb
|
PT = Prevailing Torque
|
FR = Free Running
|
||||||
Test Format
Metal Coatings' coating systems were not included in the initial testing. Following completion, MCII was able to work with the Army to duplicate the test. The information accumulated in this study gave test data that was essential to military engineers to ensure integrity of fastened joints. It also allowed the Army to standardize on one or two finishes and maximize economies of scale for cost savings.
Due to the versatility of cadmium, the study contained many different tests concentrating on those advantages. Because the most imperative applications for cadmium are fasteners, a ½-13 Grade 8 bolt was chosen for testing. The military currently uses both free running (FR) and prevailing torque (PT) nuts in various applications, so both nuts were used in the testing. The FR hex and hex flange nuts were Grade 8. The PT hex nuts (all metal type) were Grade C; the PT flange nuts (all metal type) were Grade G. The hex nuts were used for torque-tension testing, whereas the hex flange nuts were used for corrosion testing.
The aluminum used was AA 5083, which was selected to duplicate military armor. The bars were cut and machined to the dimensions 18 × 2.5 × 1.38 inches. Each bar had twelve holes machined into it: six to be used for FR nuts, and the other six to be used for PT nuts. The bolt hole diameter was 0.525 ±0.002 inch, and a chromate conversion coating per MIL-C-5541 was applied prior to fastener installation to provide a visual basis from which to determine pitting corrosion following testing. The bars were machined with a 15 degree angle on the ends, so the bars would be aligned properly in the accelerated corrosion chamber when installed on 2 × 4 inch boards.
Torque-Tension. There have been many documented occurrences of fasteners being loosened or missing from vehicles, which ultimately resulted in damage to property or injury to persons involved. Joint integrity is a vital concern when assembling vehicles, especially those being used by the armed forces.
Each fastened joint has its own dynamics, and critical joints need to be installed to the proper clamp load in order to hold an assembly together. This clamp load is obtained by applying torque to a fastener to use its tensile strength. Most assembly operations have a designated torque range to follow when installing fasteners. This controls the clamp load in a joint. Friction is the main obstacle in obtaining proper clamp load. Different finishes vary in their degree of lubricity. For example, one finish may not reach clamp load within a certain torque range, while another finish with a very low K-factor may cause the bolt to meet clamp load, stretch or even break. Low and consistent torque-tension characteristics are essential to obtain proper clamp load in every fastener that is installed.
As the initial study indicated, generic torque charts are inadequate to compensate for the numerous finishes and lubricants that are used. As a result of this inadequacy, questions arose about the quality of fastened joints. A testing benefit is that the military torque charts can be used as the acceptance criteria. As these qualities are met, standard assembly procedures can remain constant while the finish and corresponding performance change.
|
80 Cycles
|
120 Cycles
|
|||
| Finish |
PT
|
FR
|
PT
|
FR
|
| Dacromet 500® B |
73.5
|
68.8
|
82.3
|
92.2
|
| Zinc + Yellow |
126.7
|
226
|
91.7
|
81
|
| Tin Zinc |
Not Tested
|
126.7
|
96.7
|
|
| Cadmium + Wax |
117.7
|
107.2
|
95
|
106.3
|
| Cadmium + Yellow |
139.5
|
78.3
|
95
|
81.6
|
| Dacromet 320® L |
62.8
|
65.5
|
72.4
|
76.6
|
| Geomet® L |
59.2
|
68.7
|
76.3
|
72.6
|
| Coating 7 |
Not Tested
|
64.2
|
66.2
|
|
| Coating 6 |
52.5
|
54.8
|
64.2
|
64.5
|
| Dacromet 320® XL |
40.8
|
40.7
|
48
|
50.2
|
| Geomet® XL |
33.7
|
30.7
|
51.3
|
49.5
|
|
PT = Prevailing Torque
|
FR = Free Running
|
|||
Test format was per SAE J174 for the FR applications and IFI 100/107 for the PT applications. The testing took place in MCII's A2LA/ISO 9001 accredited torque-tension lab using an RS Technologies Labmaster 9504. Using a load cell, this unit is capable of measuring clamp load in a joint at different specified torques. From this data, one is able to determine if a finish has the lubricity to ensure a quality joint. In order to register consistency, 12 bolts of each finish were tested, and the mean data was used to determine the final rating. From data points received from this testing, K-factor (nut-factor), standard deviation, average clamp load and torque range at 90 ft-lb can be determined. The plus/minus three-sigma data spread was used to ensure that the clamp load would be statistically reproducible.
Corrosion Protection. The validity of the salt spray test (per ASTM B117) as an indicator of real world performance has long been questioned. Many assert that it is effective if used as a quality control tool, but it does not have a high correlation to real-life performance. Parts that are put into service are rarely kept in a wet, high salt, controlled-temperature environment, rather parts are exposed to environments that include salt mist, humidity, dry and hot conditions.
An accelerated corrosion test that has a high correlation to real-life exposure is General Motors GM 9540P, which includes the previously mentioned, more realistic cycles. General Motors uses the duration of 80 cycles to equate to 10 years in service, but due to the high cost and long life expectancy of military vehicles, the test duration was expanded to 120 cycles. Previous testing determined that the corrosion rates were different in different areas of accelerated corrosion chambers; therefore, the assemblies were rotated clockwise every 20 cycles to equalize the severity of testing.
| Finish |
Pitted Volume (mm3)
|
Max. Pit Depth (mm)
|
| Dacromet 320® L |
0
|
0
|
| Dacromet 500® B |
0.002
|
0.02
|
| Geomet® L |
0.320
|
0.10
|
| Coating 6 |
0.448
|
0.14
|
| Coating 7 |
2.300
|
0.20
|
| Cadmium + Yellow |
2.928
|
0.48
|
| Tin Zinc |
30.950
|
0.50
|
| Zinc + Yellow |
421.920
|
0.90
|
| Cadmium + Wax |
Not Measured
|
Not Measured
|
| Dacromet 320® XL |
Not Measured
|
Not Measured
|
| Geomet® XL |
Not Measured
|
Not Measured
|
To add greater severity yet duplicate field applications, the fasteners were installed into aluminum bars to introduce a dissimilar metal. When metals notably separate in the galvanic series are mated together, corrosion of the less noble metal will be accelerated due to the galvanic reaction. Flange nuts were selected for this portion of the test to increase the surface area. A hardened steel washer of the same finish (or cadmium if the same finish was not available) was used under the head of the bolt to protect against embedding the head into the aluminum. The reason the same finish was used was to eliminate any bi-metallic corrosion that may have occurred with other finishes.
The bolts were assembled/disassembled using a calibrated dial-torque wrench and were installed to 90 ft-lb. The same individual installed all the bolts. Once they were installed, they were checked a second time to ensure that the 90 ft-lb was maintained. The installation torque of 90 ft-lb was chosen for three main reasons: 1) To duplicate typical applications for installing aluminum armor to military vehicles; 2) To test the amount of clamp load retention (or relaxation) that may occur during exposure due to corrosion or lubricity of the fastener; and 3) To test the susceptibility to hydrogen embrittlement.
While it is not a foolproof indicator, a cost effective means to test for hydrogen embrittlement is to subject the finished part to normal installation stresses. It is only when a fastener is put in stress that failure as a result of hydrogen embrittlement will occur. This applies to both internal hydrogen embrittlement (IHE), caused as a result of the finishing process, in addition to external hydrogen embrittlement (EHE), caused by the chemical reaction occurring during corrosion. In both of these failure methods, hydrogen diffuses into the steel microcrystals and separates the once-fused steel particles.
| Finish |
Before Testing
|
80 Cycles
|
120 Cycles
|
| Cadmium + Yellow |
1
|
0.1
|
0.1
|
| Cadmium + Wax |
1
|
0.1
|
0.1
|
| Tin Zinc |
0.5
|
0.5
|
0.5
|
| Dacromet 500 ® B |
6
|
6
|
5
|
| Dacromet 320 ® XL |
10
|
10,000
|
Infinite
|
| Dacromet 320 ® L |
4
|
Infinite
|
Infinite
|
| Zinc + Yellow |
2
|
Infinite
|
Infinite
|
| Geomet® L |
4
|
Infinite
|
Infinite
|
| Geomet® XL |
10
|
Infinite
|
Infinite
|
| Coating 7 |
20,000
|
Infinite
|
Infinite
|
| Coating 6 |
Infinite
|
Infinite
|
Infinite
|
In order to quantify the amount of corrosion that occurred as a result of the bimetallic cell, the aluminum bars were measured for total pitted volume (mm3) and maximum pit depth (mm). The pitted volume was measured with the aid of a binocular-scope and various measuring devices. The pit depth was measured using a Leco microhardness tester with a calibrated height adjustment. In both of these cases, the area surrounding each hole was measured, and the average of these values was used for ranking. The same individual using the same guidelines as the initial study conducted pitting corrosion testing at United Defense.
Conductivity. Essential for grounding applications, conductivity is required in order to protect the expensive electronics used in military vehicles. In many cases, expensive specialty finishes are required to retain the conductivity essential to protect the vehicle. Cadmium displays excellent conductivity characteristics, which are retained throughout testing.
Following installation of the fasteners to the aluminum bars, an ohmmeter was used to test the conductivity. One probe was placed on the aluminum and the other was placed on two or more nuts of the finish. Readings greater than 20,000 ohms were considered infinite resistance, and the finish was deemed non-conductive. The initial readings were used as the baseline to see how the finish reacts to the corrosion testing.
Thickness. Use of standard thread sizes is an important element in qualifying a cadmium alternative. If a special fastener is required, it drives up the costs associated with the vehicles and increases tax dollars required to pay for it. Initial installation is one consideration, but when the vehicle needs to be serviced, finding the special-order replacement hardware can be especially difficult.
The thickness testing was conducted on a Fischerscope using magnetic induction. This testing requires an unfinished bolt to be used for a baseline and a flat surface (hex head or flat of a nut) on which to take a measurement. A total of three readings were taken on each part (bolt, PT hex, PT flange, FR hex and FR flange) using an average of the readings as a total thickness.
| Finish |
Bolt
|
PT Nut
|
FR Nut
|
| Dacromet 500® B |
0.382
|
0.385
|
0.380
|
| Cadmium + Yellow |
0.490
|
0.441
|
0.428
|
| Cadmium + Wax |
0.441
|
0.423
|
0.398
|
| Dacromet 320 ® L |
0.383
|
0.340
|
0.403
|
| Geomet® L |
0.528
|
0.478
|
0.535
|
| Coating 7 |
.0770
|
1.200
|
1.000
|
| Coating 6 |
.0765
|
0.746
|
0.686
|
| Tin Zinc |
Not Tested
|
||
| Dacromet 320 ® XL |
0.441
|
0.382
|
0.430
|
| Geomet® XL |
0.647
|
0.485
|
0.540
|
| Zinc + Yellow |
0.339
|
0.274
|
0.286
|
Cost Analysis. Just as the military engineering standards are acclimated to the performance of cadmium, the same is true for the purchasing system. The finish that provides the most consistent functional benefits would be eliminated if it were not cost-effective in replacing cadmium. A cost factor was included to provide engineering and purchasing a definitive test from which to gather essential information. The cost factor used cadmium as a baseline (cadmium=1) and was obtained using major sources of supply in the Detroit, MI, metropolitan area.
Finishes Tested. This study featured eleven finishes, including some lubricants to assist in assembling the PT nuts. As previously mentioned, cadmium + yellow dichromate and cadmium + wax (per Federal specification, QQ-P416, Class 2, Type 2) were used as the upper baseline for corrosion, and zinc + yellow dichromate (per ASTM B633, Class Fe/Zn 8, Type 2) was used as the lower baseline. Different variations of inorganic water-based coatings were the focus of this test study due to their large demand worldwide in applications similar to those targeted for replacement.
The Dacromet® and Geomet® coating systems are water-based Zn/Al coatings with varying degrees of lubricity. The Dacromet coating systems (applied per Ford S301, DaimlerChrysler PS 5873L and PS 9666) contain chrome and have been used in automotive and military applications as cadmium replacements. There are differences in the Dacromet coating systems tested. Dacromet 500® contains an integral lubricant in the basecoat, Dacromet 320® L uses the Plus® L lubricated sealer, and Dacromet 320® XL uses the Plus® XL lubricated sealer.
The Geomet coating systems (applied per DaimlerChrysler PS 5873L, PS 9666 and GMW 14) share a chrome-free base coat, which is the leading development in the finishing industry to eliminate chrome in vehicles. Geomet L and Geomet XL use the same chrome-free Plus L and Plus XL sealers to provide lubricity and additional corrosion protection. Coatings 6 and 7 are solvent-based organic coatings, which contain hexavalent chrome (6) and are hexavalent chrome free (7).
Finally tin zinc, currently used for applications requiring moderate corrosion protection and conductivity, was tested. All finishes were applied in industrial, bulk finishing operations to obtain real-world performance.
Test Results
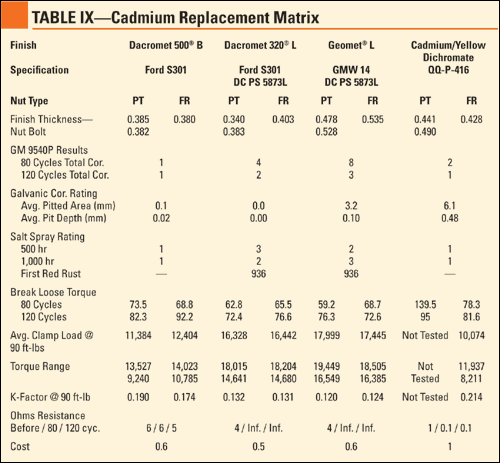
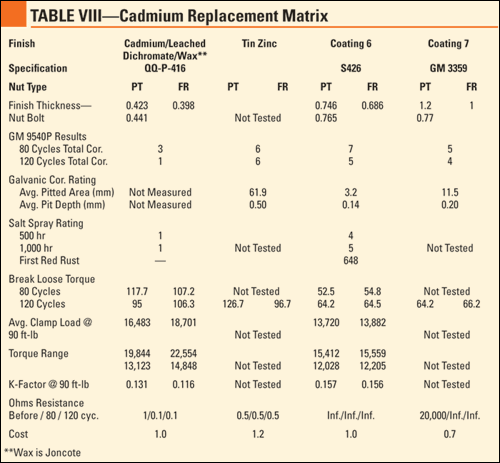
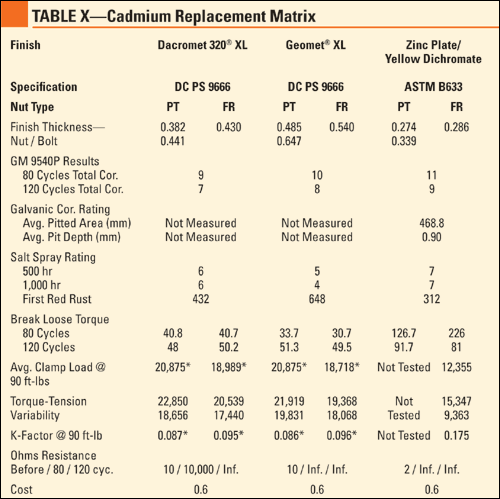
Torque-Tension Results. The information obtained from the torque-tension testing included the torque range, average clamp load and K-factor at 90 ft-lb. This information is essential in planning fastened joints because there must be statistical confidence out of numerous bolts that the proper clamp load is achieved and retained. In the initial study, some of the finishes tested required supplemental lubricants to achieve the desired K-factor of 0.13-0.15, but in the "dry" state these finishes reacted similar to the previous study. Table 1 shows the results of the torque-tension testing.
Corrosion Protection Results. Following exposure to 120 cycles of GM 9540P, the assemblies were rinsed in warm water, allowed to dry and rated according to cosmetic corrosion, break-loose torque and galvanic corrosion. According to the GM 9540P test method, bare steel coupons were tested to provide a benchmark with regards to severity. The corrosion of these coupons was 63% higher than the test parameters, indicating the parts were exposed to an extremely severe environment.
Cosmetic Rating. All of the finishes were compared to each other regarding the amount of red/white corrosion on the nut and threads of the bolt. The best and worst finishes remained constant throughout the testing, but the finishes in the middle changed positions. Table 2 shows the cosmetic corrosion rating of the different finishes.
Break-Loose Torque. Following cosmetic rating, the bolts were disassembled, and the break-loose torque was recorded. The target break-loose torque was 90 ft-lb, which indicates clamp load retention throughout the test, although variations were expected due to the severity of the exposure. Due to white or red corrosion on the tin zinc parts, the break-loose torque was higher than expected. Table 3 shows the results of the break-loose torque testing.
Galvanic Rating. Following removal of the fasteners, the aluminum bars were sent to United Defense for pitting corrosion evaluation. The intent for the pitted volume measurement was to include only the surface area of the pits and not the non-corroded areas between the pits. The pit depth was measured using the microscopic method, by focusing on the top of the sample and refocusing on the bottom of the pit while measuring the vertical height change. With regards to relative performance, the results were consistent with the previous study. Table 4 describes the results of the galvanic rating.
Conductivity Results. Following exposure, the assemblies were tested to explore the effects of the corrosion testing on the conductivity of the finish. Four finishes were found to retain their conductivity throughout 120 cycles. Others exhibited acceptable conductivity at the beginning of the testing, but lost those attributes by completion of the test. Table 5 outlines the conductivity test results.
Thickness Results. Bolts of each finish were set aside in order to conduct thickness testing to ensure that the thickness was at least at the specified minimum, but not excessively more, which would result in problems mating a nut to a bolt. Although the thickness of the pitch and root of the threads cannot be determined using this method, there is a direct correlation between the tested areas and the pitch/root thickness. Table 6 describes the thickness associated with each of the finishes tested.
Cost Analysis Results. In order for testing to be beneficial, the qualified finishes must be readily available in the market at a cost-effective price. A cost factor was determined using cadmium as a baseline, so engineers would know the relative price increase/decrease compared with current cadmium plated fastener purchase. Table 7 shows the cost factor using the cost of cadmium as 1.
The testing concluded that Dacromet 500 B could be used as a direct cadmium replacement for all engineering applications. This coating system performed well in all of the categories tested, offering excellent corrosion protection in a very thin film. The galvanic corrosion rating was superior to cadmium with the cosmetic corrosion rating equaling the performance of cadmium. The clamp load retention was comparable to the cadmium baseline; however, the torque characteristics were more lubricious. The lubricity was not sufficient to meet the specified criteria, so the addition of a dry film lubricant to the coated nuts is required. Dacromet 500 B also retained its conductivity (although it was slightly less than tin zinc) and reduced the cost of hardware by 40% compared to cadmium.
Dacromet 320 L and Geomet L performed better than cadmium with regards to galvanic corrosion, reducing the amount of pitted volume and maximum pit depth. The cosmetic corrosion protection was somewhat inferior to cadmium, but the difference was negligible considering the severity of the test. The torque characteristics of these coating systems, which use the same topcoat, were within the acceptable range according to the Army torque charts with regards to both prevailing torque and free running applications. The weakness that was exposed through this testing was that the conductivity measured before exposure was not retained throughout the test duration. At 50% and 60% the cost of cadmium, respectively, these coatings can be deemed as cadmium replacements for some applications, but not as a drop-in replacement in all engineering areas.
Coatings 6 and 7 performed better than zinc plate + yellow chromate; however, they do not offer superior corrosion resistance. Coating 7 showed worse galvanic corrosion ratings than the water-based coatings with regards to both pit depth and pitted volume. Also, the torque characteristics of Coating 6 indicate that a supplemental lubricant is necessary to meet the torque characteristics specified by the Army. The same is expected from Coating 7 due to the close resemblance of the topcoat, but testing was not completed in this research study. Another weakness that was originally exposed in the previous study was that these coatings do not allow an electrical current to be passed through the substrate even at the beginning of testing. Another concern for guaranteeing joint integrity is the reduction of the clamp load after exposure to testing. With a 28% reduction between the installation torque and break-loose torque, careful attention must be paid in assembly. At twice the thickness of cadmium, functionality in mating a nut to a bolt could potentially be a problem with the expectation that the threads would have to be undersized in order to accommodate the thickness. At the same or slightly lower cost than cadmium, these coatings can be characterized as acceptable alternatives with regards to corrosion protection, but consideration must be made for the torque characteristics and the health hazards associated with their application.
Tin-zinc provided mediocre performance in all areas except conductivity, in which it performed second only to cadmium. White corrosion products were apparent very early in the testing, which lead to somewhat higher break-loose torque values following testing. These corrosion products also showed that there was a galvanic reaction when mated with the aluminum, resulting in considerable pitting. At a cost 20% higher than cadmium, tin-zinc is recommended for applications that require conductivity, but not in areas that involves contact with aluminum due to the bimetallic reaction.
Dacromet 320 XL and Geomet XL outperformed zinc + yellow dichromate, but were rated near the bottom of the cosmetic corrosion ranking. The lubricity of each of these coating systems, which share the same sealer, was too low. When measurements were attempted at the standard 90 ft-lb, bolts were broken. The parameters were changed slightly to 75 ft-lb to obtain valuable data from the testing. The coating thickness was comparable to cadmium; however, bolts did not perform well with regards to break-loose torque or conductivity. At 60% the cost of cadmium, these coatings may be chosen selectively for applications requiring a low K-factor to ease the installation of large fasteners.
The results of this study show inherent benefits and weaknesses of the finishes tested. The Dacromet 500 B was the only finish that performed equivalent to or better than cadmium in all applications. The other benefits it offers is that it is VOC compliant and is cheaper than cadmium. With results and properties similar to Dacromet 500 B, Dacromet 320 L and Geomet L are viable options for many applications as a cost-effective environmentally compliant alternative to cadmium. The other finishes tested have weaknesses just as many previously tested options do, but still can not be accepted as drop-in replacements.
The authors would like to acknowledge the following individuals for their contributions: Garry Carda, AM General Corporation; Mike Kardasz, General Fasteners Company; and Lauren Lanier, United Defense.