Experimental Study on the Formation of Electroless Nickel-Boron Coatings from a Borohydride-Reduced Bath on Mild Steel
This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 13, 2012.
#research #nasf #surfin
by Véronique Vitry, Adeline Sens and Fabienne Delaunois, Service de Métallurgie, Université de Mons,Mons, Belgium
This paper is a peer-reviewed and edited version of a presentation delivered at NASF SUR/FIN 2012 in Las Vegas, Nev., on June 13, 2012.
Featured Content
ABSTRACT
The quality and homogeneity of electroless nickel-boron coatings are very important for applications in corrosion and electronics and are completely dependent on the formation of the deposit. The formation process of electroless nickel-boron was investigated by immersing steel samples in an unreplenished bath for various periods of time (from 5 sec to 1 hr). The coatings obtained at the different stages of the process were then characterized. Thickness was measured by SEM, morphology was observed, weight gain was recorded and the composition of the coatings was obtained from GD-OES. Three main phases were identified during the coating formation and links between plating time, instantaneous deposition rate, chemistry of last formed deposit and morphology were established. The mechanism for the initial deposition on steel substrates for a borohydride-reduced electroless nickel bath was also observed. Those results were related to chemical evolution in the unreplenished plating bath during the process. This shed light on the phenomena occurring in the plating bath and their influence on coating formation.
Keywords: electroless nickel-boron, borohydride bath, deposition mechanism, electroless deposition, steel substrate, corrosion resistance, electronics applications.
Introduction
Developed in 1946 by Brenner and Riddel,1 electroless nickel deposition is now a well-established surface engineering process that is widely used because of its properties, such as uniform thickness, high hardness and good corrosion resistance.2-5
While nickel-phosphorus deposits are more popular, both in industrial applications and research, electroless nickel-boron coatings are not without interest, because they offer a higher hardness, as well as promising electrical and tribological properties.6-14 They are consequently used in several industrial domains, including aeronautics, the petrochemical industry, the food industry, firearms, etc.
The research emphasis on electroless nickel usually involves either optimization of plating parameters, investigation of coating properties or the effect of heat treatments. This is particularly true in the case of nickel-boron and the papers dealing with deposit formation are few and far between.7,8,15,16 However, it is undeniable that the initiation and growth of the coating influences its properties. It has been shown by Rao, et al.17 that the structure and chemistry of electroless nickel-boron coatings obtained in unreplenished and non-agitated baths was heterogeneous over the thickness and varied during deposition. In her very detailed work, Clerc7 observed deposit growth, also in unreplenished and non-agitated baths, and identified three growth phases. Those works, probably the most detailed on the subject, were carried out in non-agitated baths, which are very different from industrial conditions (agitated and replenished solution).
Moreover, the influence of substrate on the initial stages of coating formation, while widely investigated in the case of nickel-phosphorus,18-27 is still a largely uninvestigated area of electroless nickel-boron plating. This is unfortunate, knowing the catalytic nature of the electroless plating process.
During plating, the newly formed nickel alloy provides the catalytic surface needed for the continuing reaction,28,29 but the initiation of electroless deposition (i.e., the formation of the first continuous layer of nickel on the substrate) can be brought about in three known ways: (1) spontaneous catalytic activity of the substrate for the oxidation of the reducing agent, (2) a displacement reaction between an easily oxidizable substrate and the nickel ions present in the solution or (3) activation of the substrate in a solution of catalytically active metal salts (like palladium) or by galvanic coupling.4 In the absence of catalytic activation by galvanic coupling or by catalyst salts immersion, the actual initiation mechanism for electroless deposition on a given substrate is rarely known or investigated.
In this paper, we observe and describe the initiation mechanism for electroless nickel-boron on mild steel, as well as the initiation and growth of the coating. The links between local chemistry, coating morphology, and, when possible, coating properties are closely investigated. Variations in the growth rate of the deposit during the plating process are also examined. Finally, the influence of batch replenishment on coating chemistry and morphology is also discussed.
Experimental details
Sample preparation
The mild steel chosen for this study was St-37 sheet coupons (1 mm thick). The size of samples varied from 2 × 2 cm to 10 × 10 cm. However, the total surface of samples present in the bath was kept at 200 cm2 at all times, leading to a constant bath load of 25 cm2/L.
Samples were prepared before plating by mechanical grinding (up to 4000 grade SiC paper30), acetone degreasing, acid etching (activation) in 30% hydrochloric acid, and a deionized water rinse. Plating was carried out with an electroless nickel-boron bath developed by Delaunois, et al., which has been described extensively elsewhere.31,32 The bath was reduced with sodium borohydride and stabilized with lead tungstate and operated at 95 ± 1°C. The plating cell was an 8-liter Teflon-coated thermostable bath. Immersion times varied from 5 sec to 60 min, as shown in Table 1. To avoid the influence of reactant consumption and product build-up on initiation of the process, the plating solution was completely renewed after each experiment. Samples were rinsed with running deionized water and dried in hot air directly after plating.
The influence of bath replenishment was investigated by plating samples for 90 min, with the addition of fresh reactant 30 min after the beginning of the plating process. Reactant additions were calculated from repeated chemical analyses of bath chemistry during use, that were compiled in a database.33
The initiation mechanism was determined with the help of a specially-designed experiment. Samples were immersed for 4 min in a plating bath free of reducing agent.
Table 1 - Deposition time for the observation of the initiation of electroless deposits.
Sample # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Deposition Time, sec | 5 | 15 | 30 | 60 | 90 | 240 | 420 | 600 | 1800 | 3600 |
Deposition Time, min | 0.083 | 0.25 | 0.50 | 1.0 | 1.5 | 4.0 | 7.0 | 10.0 | 30.0 | 60.0 |
Deposit analysis
The initiation mechanism, nucleation sites and morphological evolution of the deposit were investigated by scanning electron microscopy (SEM), with a Jeol JSM 5900LV scanning electron microscope. Cross sections were ground to a mirror polish using a diamond paste (1/4 micron) and then etched using 10% nital before SEM observation. Qualitative evaluation of the colonization of the substrate by the deposit was investigated by EDX analysis. The nickel amount detected on the surface (under otherwise identical conditions) increases with nickel coverage and deposit thickness until the coating is continuous and thick. To allow comparison between various samples, a surface of 1 mm2 was analyzed at a magnification of 100× for 60 sec. The link between local variations of the chemistry and morphology was made by the combined use of SEM imagery and chemical analysis, by ICP (after dissolution in aqua regia, with the use of a Thermoelectron ICP Iris Intrepide 2 - Dual View apparatus) for the average composition and by GD-OES (using a Horiba-Jobin-Yvon GD-Profiler 2 apparatus, calibrated for quantitative analysis).
Results and discussion
Initiation mechanism
There are only two possible mechanisms for the beginning of the deposition process for electroless nickel-boron on an un-catalyzed steel substrate: (1) the formation of an ultra-thin layer of nickel by displacement (redox reaction between nickel ions and the substrate, accompanied by dissolution of the substrate material, without intervention of the reducing agent), and (2) oxidation of the sodium borohydride due to catalytic activity of the substrate (steel) and subsequent reduction of nickel salts that form a very thin nickel layer.
It is not possible to distinguish which mechanism is responsible for the formation of the first nickel layer during plating as both cause similar end results. However, using a bath without reducing agent (all other conditions kept identical to plating conditions) allows this identification. The formation of the first nickel layer by displacement does not need any intervention from the reducing agent. If this mechanism is responsible for initiation, nickel should be detected by EDX on the surface after immersion in the borohydride-free bath.

Figure 1 - Surface observation of a sample immersed for 4 min in a bath without reducing agent.
The surface of a sample immersed in a reducer-free bath for 4 min. is shown in Fig. 1. No nickel was detected on this sample, but some lead crystals were observed on surface defects in the coating. This allows us to exclude nickel displacement as the initiation mechanism for electroless deposition and to affirm that catalytic oxidation of borohydride by the substrate is the main factor for deposit initiation. The formation of lead crystals can however be attributed to a displacement reaction between iron and lead, which is not really surprising because the redox potential of lead is higher than those of nickel and iron (-0.47 V for Fe+2/Fe; -0.27 V for Ni+2/Ni; -0.13 V for Pb+2/Pb).
Morphological observation of deposit initiation and formation on mild steel.
Surface observation of coatings at various stages of the process
Immersions shorter than 15 sec in the plating bath brought no evidence of nickel deposition detectable visually by SEM. However, some nickel was detected by EDX analysis after 15 sec, as shown in Table 2. This allows us to determine the induction period to be in the 5 - 15 sec range. After 30 sec, evidence of nickel deposition is easily observed in the form of nodules that can be found preferentially on the scratches and surface defects of the substrate, as shown in Fig. 2a. At this time, the nickel layer is not continuous and the size of the nodules stays between 0.1 and 0.2 µm. The EDX results, indicating 20% of nickel in the top 1 µm, suggest that nickel may also be detected outside of the observable nodules. This is in complete agreement with the EDX results obtained after 15 sec. After 60 sec (Fig. 2b), the sample surface is fully colonized and the nodules are larger. However, the nickel layer is not continuous.
Table 2 - Results of EDX analysis at the beginning of the plating process.
Time, sec | 5 | 15 | 30 | 60 | 90 | 240 |
Fe, at% | 100 | 94.3 | 81 | 42 | 26 | 7 |
Ni, at% | 0 | 5.7 | 19 | 58 | 74 | 93 |
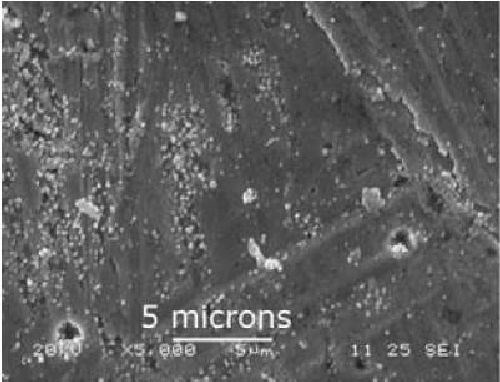

Figure 2 - Surface of mild steel (St 37) samples after an immersion of (a) 30 sec and (b) 60 sec.
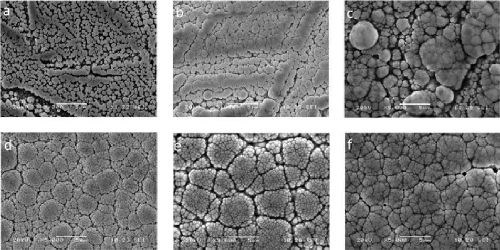
Figure 3 - Surface of mild steel (St 37) samples after an immersion of (a) 90 sec, (b) 4 min (240 sec), (c) 7 min, (d) 10 min, (e) 30 min and (f) 1 hr in the nickel-boron deposition bath.
Thickening of the nodules and leveling of the surface was observed after an immersion of 90 sec to 4 min (Figs. 3a and 3b). The small residual amount of iron detected by EDX after 4 min indicates that the coating is continuous and nearly densified at this point. After 7 min (Fig. 3c), a refinement of the size of the surface nodules was observed. This refinement continues up to 10 min (Fig. 3d) and is followed up to 30 min by a slight thickening of the columns accompanied by leveling of the coating (Fig. 3e). After 1 hr, the intercolumnar spaces appear to be nearly filled out and the apparent size of the nodules is quite small (Fig. 3f).
We were able to observe that the size of the surface nodules was not constant during deposition. This suggests that the deposition process is a succession of germination phases (during which small nodules are formed), and growth phases (during which the formed entities become larger).
Cross section observation of coatings at various stages of the process
It was not possible to observe coatings by SEM on cross section for plating times shorter than 90 sec. The first observable sample, at 90 sec (Fig. 4a), presented a very thin continuous layer of nickel close to the interface, over which nodules of various sizes were growing. This corroborates the surface observation and suggests at least two layers of growth were already present at this time. After 4 min, the balloon-like nodules were packed together and the coating was much denser (Fig. 4b), with a thickness close to 1 µm. After 7 min (Fig. 4c), the lateral growth of the nodules was no longer possible and a columnar morphology began to form. A phenomenon akin to secondary germination induced the separation of the column into several smaller branches, leading to refinement of the upper part of the coating. After 10 to 30 min (Figs. 4d and 4e), the refinement was still observed and was accompanied by a densification of the coating, with a closing of the more important porosities. The resulting coating (Fig. 4f) appeared as a superposition of layers of columns generated by alternating refining and lateral growth phases of the initial nodules.

Figure 4 - Cross-section observation of mild steel (St 37) samples after an immersion of (a) 90 sec, (b) 4 min (240 sec), (c) 7 min, (d) 10 min, (e) 30 min and (f) 1 hr in the Ni-B plating bath.
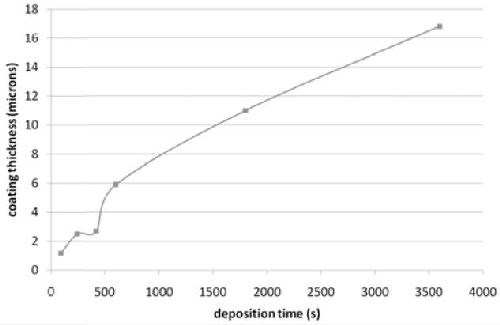
Figure 5 - Evolution of deposit thickness with immersion time (on polished St 37 steel).
To assess the growth rate, thickness of the deposit was measured on SEM micrograph, as shown in Fig. 5. After an initial phase of quick growth, leading to the formation of a loosely-packed ensemble on balloon-like nodules, the thickness increased very slowly during the densification phase (4 to 7 min). As soon as the columnar morphology was formed, the thickness increased nearly linearly up to 1 hr of plating, with an average growth rate of 1 µm/hr.
Chemical heterogeneities
The average composition of the electroless nickel-boron coatings was obtained from ICP analysis and was as follows: 6 wt% B, approximately 1 wt% Pb and 93 wt% Ni.
Profile composition, obtained from GD-OES analysis, is shown in Fig. 6, superimposed with a SEM image of a cross section of the same deposit. The nickel and boron content in the deposit fluctuate only slightly across the deposit, with a boron content very close to the 6 wt% detected by ICP. This indicates that the decrease of reactant concentration expected during a non-replenished (but agitated) electroless plating process does not strongly influence the chemistry of the coating. This is an important feature because important variations have been reported by Rao, et al.17 in the case of non-agitated baths.
The amount of lead detected by GD-OES stays in the 0.25 - 0.45 wt% range at all times. However, the fluctuations of this parameter are much more marked than those of the Ni and B content. There is a high lead content (close to 0.45 wt%) close to the substrate-coating interface. There is then a quick decrease in the first 2 - 3 µm of the coating. The lead content then stays low in most of the coating but increases near the top surface (at the end of the deposition process).
There is an observable link between lead content fluctuations and morphology. When the lead content is lower (in the middle of the coating), the columns are wider, while smaller columns are observed at the beginning and the end of the process, when the lead content is higher.
Lead content fluctuations can also be linked with the growth rate, as the lowest lead concentration can obviously be matched with a quick growth phase observed after 7 min of plating and the highest concentration (at the beginning of the process) coincides with the episode of very slow deposition observed at earlier times, during the densification of the initial balloon-like nodules. The important amount of lead detected near the interface is probably related to the very high specific surface of the balloon-like formation, which is favorable to lead adsorption. The growth of the coating is then slowed by the presence of a huge amount of stabilizer at the reaction surface and by the diffusion phenomena needed to bring reactant between the existing nodules.
The lower plating rate observed at the end of the plating process, that is usually attributed to reactant depletion, is probably also influenced by the higher lead content detected near the surface of the sample.
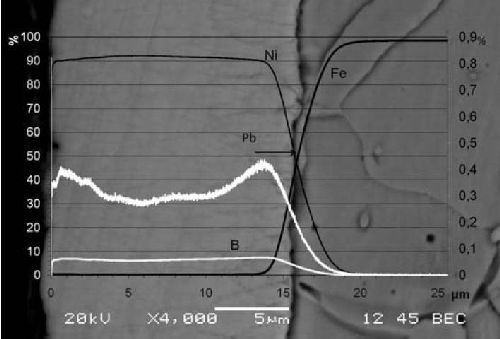
Figure 6 - Comparison of the composition (from GD-OES) and morphology (from SEM) across the deposit.
Influence of batch replenishment on coating morphology and chemistry
Batch replenishment of the bath (i.e., the addition of reactant at a given time during the plating process) has a visible influence on coating morphology, as can be seen in Fig. 7. There is a clear demarcation line in the coating between the material deposited before and after the addition of the reactant. The deposit resembles what would be obtained if two distinct plating episodes had been carried out on the substrate.30 This means that batch replenishment induces a new episode of high density germination. This is promising information for the corrosion resistance of electroless nickel-boron coatings. The weak point in this matter is the presence of porosity throughout deposit, which can be greatly reduced by the “double coating” effect brought by batch replenishment.
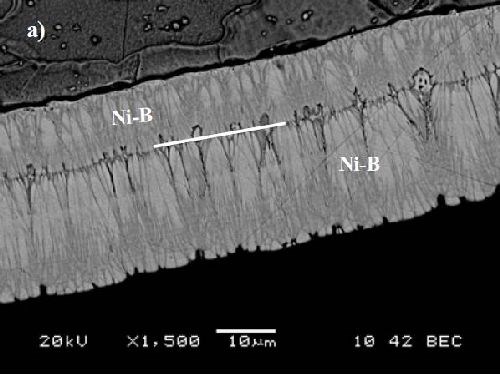
Figure 7 - Effect of batch replenishment on the morphology of the coating (cross section SEM image).
Due to the very significant effect of batch replenishment on the coating morphology, an in-depth chemical analysis was also carried out on the replenished samples. As can be seen in Fig. 8, the lead content evolution in the coating is smooth. However, this could be due to the leveling effect of GD-OES analysis. The evolutions at the beginning (before replenishment) and at the very end of the plating process (the last 5 µm deposited) are similar to what is observed in the case of unreplenished baths. In the middle part however, there is first (coming from the substrate / coating interface) an increase of lead content that could be attributed to replenishment, in a way similar to the higher lead content observed at the beginning of the plating process, then a slow decrease until the final increase in the last 5 µm. The boron content appears to be slightly lower in the materials deposited after the replenishment (the top 15 µm), but the measured content is stable during both deposition phases, as if two different coatings had been deposited on top of each other without mutual influence.
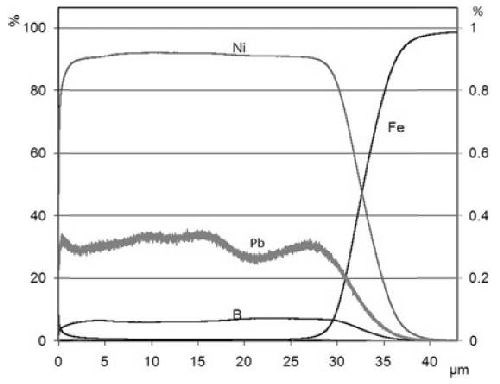
Figure 8 - GD-OES in-depth chemical analysis on a sample with batch replenishment.
Conclusion
In the present work, the mechanism responsible for the initiation of electroless nickel-boron plating on st-37 steel has been identified, catalytic oxidation of sodium borohydride on the active substrate. This has been proven by the inability, under actual plating conditions, for the nickel-iron displacement reaction (which is the other possible mechanism) to occur.
The different phases of the plating process were also identified:
- A short induction period before any nickel is deposited, followed by a period during which some is deposited, but not yet in the form of observable nodules.
- The germination, and then growth of nodules, which take a balloon-like shape.
- The densification of the coating by filling of the voids between the nodules and the transition to a columnar morphology (after 4 min of immersion). During this phase, the thickness of the coating does not increase.
- Several growth and refinement phases of the columns, the first of which happens after 4 to 7 min, that can be linked to the lead content of the last deposited material.
The morphology of the coating is very much influenced by chemical heterogeneities, as wider columns are observed where the lead content is higher.
Batch replenishment of the plating solution induced a new phase of germination and growth that could, in a morphological and chemical way, be considered as the deposition of a separate coating on top of the first one. Both layers (the one deposited before and the one deposited after replenishment) have very similar features and can be compared to coatings deposited in a non-replenished bath.
This work brings forth considerable information about the formation of electroless nickel-boron coatings on mild steel substrates. However, some aspects and phenomena remain unexplained. Further investigation, mainly about structural evolution of the coating, is thus underway, as well as a study of electroless plating initiation on various steel substrates.
Acknowledgements
The authors wish to thank Mr. J. Dutrieux from INISMA for its help with the SEM work, Dr. F. Renaux from Materia Nova for the XPS analysis and the University of Udine for the GDOES analysis.
References
1. A. Brenner & G. Riddel, J. Res. Nat. Bur. Stds., 37, 31 (1946).
2. K. Hari Krishnan, et al., Met. and Mat. Trans. A, 37 (6), 1917 (2006).
3. P. Peeters, et al., Electrochim. Acta, 47 (1-2), 161 (2001).
4. A. Riedel, Electroless Nickel Plating, Finishing Publications Ltd., London, 1991.
5. R. Elansezhian, B. Ramamoorthy & P. Kesavan Nair, J. Mater. Process. Technol., 209 (1), 233 (2009).
6. T. Watanabe & Y. Tanabe, Trans. Japan Inst. of Metals, 24 (6), 396 (1983).
7. M-A. Clerc, Ph.D. Thesis, Besançon (France), 1986.
8. P.S. Kumar & P.K Nair, Nanostruct. Mater., 4 (2), 183 (1994).
9. A. Chiba, H. Haijima & W.C. Wu, Ultrasonics, 42 (1-9), 617 (2004).
10. A. Mondal, et al., Mater. Res. Bull., 39 (14-15), 2187 (2004).
11. Y.W. Riddle & T.O. Bailer, JOM, 57 (4), 40 (2005).
12. K. Krishnaveni, T.S.N. Sankara Narayanan & S.K. Seshadri, Surf. & Coat. Tech., 190 (1), 115 (2005).
13. S. Ziyuan, W. Deqing & D. Zhimin, Appl. Surf. Science, 221 (1-4), 62 (2004).
14. T.S.N. Sankara Narayanan & S.K. Seshadri, J. Alloys & Compounds, 365 (1-2), 197 (2004).
15. V. Vitry, A-F. Kanta & F. Delaunois, Mater. Sci. Eng B, 175 (3), 266 (2010).
16. V. Vitry, A-F. Kanta & F. Delaunois, Praktische metallographie, 42, 315 (2010).
17. Qun-li Rao, et al., Appl. Surf. Science, 240 (1-4), 28 (2005).
18. L. Li & M. An, J. Alloys & Compounds, 461 (1-2), 85 (2008).
19. S. Karmalkar & V.P. Kumar, J. Electrochem. Soc., 151 (9), C554 (2004).
20. H. Jha, et al., Materials Letters, 63 (16), 1451 (2009).
21. A. Duhin, et al., Electrochim. Acta, 54 (25), 6036 (2009).
22. L. Li, M. An & G. Wu, Applied Surf. Sci., 252 (4), 959 (2005).
23. T. Homma, et al., J. Electrochem. Soc., 144 (12), 4123 (1997).
24. H. Matsubara, et al., Electrochim. Acta, 47 (25), 4011 (2002).
25. H. Matsubara, et al., Electrochim. Acta, 52 (2), 402 (2006).
26. Z. Liu & W. Gao, Surf. Coat. Tech., 200 (11), 3553 (2006).
27. H. Matsubara, et al., Electrochim. Acta, 52 (9), 3047 (2007).
28. B.J. Hwang & S.H. Lin, J. Electrochem. Soc., 142 (11), 3749 (1995).
29. G.O. Mallory & J.B. Hajdu, Electroless Plating: Fundamentals and Applications, AESF, Orlando, FL; NASF, Washington, DC, 1990; pp. 1-56.
30. A-F. Kanta, V. Vitry & F. Delaunois, J. Alloys & Compounds, 486 (1-2), L21 (2009).
31. F. Delaunois & P. Lienard, Surf. & Coat. Technol., 160 (2-3), 239 (2002).
32. F. Delaunois, et al., Surf. & Coat. Technol., 124 (2-3), 201 (2000).
33. V. Vitry, Ph.D. Thesis, UMONS, Mons (Belgium), 2010.



RELATED CONTENT
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Smut and Desmutting
Question: I am new to this industry and have heard about smut and desmutting operations.
-
Gold and Silver Plating Basics
An overview of precious metal electroplating processes.


















