Published
Growth of ZnO Nanorod Arrays on Flexible PDMS Substrates
In this work, well-aligned zinc oxide (ZnO) nanorods were successfully deposited on flexible polydimethyl-siloxane (PDMS) substrates by a simple hydrothermal growth method. The low temperature (<100°C) synthesized ZnO nanorods were investigated by scanning electron microscopy (SEM), x-ray diffraction (XRD) and photoluminescence (PL) spectroscopy. The results indicate that the average diameter of a ZnO nanorod was 161±10 nm. The highly crystalline and uniform ZnO nanorods on flexible PDMS show luminescent properties with an emission wavelength at 450 nm. It is anticipated that the low temperature deposition of ZnO on PDMS may have significant potential application in flexible optical devices.
by Yi Chen and Jin Zhang
Department of Chemical and Biochemical Engineering, University of Western Ontario
London, Ontario, Canada
ABSTRACT
In this work, well-aligned zinc oxide (ZnO) nanorods were successfully deposited on flexible polydimethyl-siloxane (PDMS) substrates by a simple hydrothermal growth method. The low temperature (<100°C) synthesized ZnO nanorods were investigated by scanning electron microscopy (SEM), x-ray diffraction (XRD) and photoluminescence (PL) spectroscopy. The results indicate that the average diameter of a ZnO nanorod was 161±10 nm. The highly crystalline and uniform ZnO nanorods on flexible PDMS show luminescent properties with an emission wavelength at 450 nm. It is anticipated that the low temperature deposition of ZnO on PDMS may have significant potential application in flexible optical devices.
Keywords: zinc oxide (ZnO), aligned, nanorod, polydimethyl-siloxane (PDMS), hydrothermal
Introduction
The zinc oxide (ZnO) nanorod is an emerging one-dimensional nanostructured semiconductor with a wide band-gap, Eband gap = 3.37 eV. One-dimensional ZnO nanomaterials have been considered the most efficient potential material for ultraviolet (UV) light emitting diodes, field effect transistors (FET), chemical sensors and solar cells due to their special electrical, optical, chemical and piezoelectric properties.1-3 Most recently, ZnO nanomaterials also show potential in the fields of biomedicine and biosensors because of their bio-inert properties.4,5
Various methods have been developed to produce one-dimensional ZnO nanomaterials, including sol-gel methods, polymer-assisted growth, template-induced growth, solution-liquid-solid growth in an organic solvent, metal-organic chemical vapor deposition (MOCVD) and hydrothermal synthesis.6-10 Compared to most of the processes, the hydrothermal technique has many advantages over other techniques, e.g., a relatively simple process, low synthesis temperature, controllable size and low equipment cost. Various shapes of ZnO nanomaterials have been reported using hydrothermal synthesis such as nanorods (nanowires), nanobelts and nanoparticles.11,12 Current interest lies in producing well aligned ZnO nanorods for electrical and optical device applications.13,14 In this work, we prepared aligned ZnO nanorod arrays by using the simple hydrothermal process. Polydimethyl-siloxane (PDMS) was used as a substrate material because of its advantages in terms of flexibility, low cost, chemical stability and transparency. Characteristics of ZnO nanorod arrays were assessed by scanning electron microscopy (SEM), x-ray diffraction (XRD) patterns and photoluminescence (PL) spectroscopy.
Experimental details
Polydimethyl-siloxane (PDMS) substrates were first fabricated. Five grams of PDMS* was mixed with a curing agent** in a 10:1 ratio. After stirring the mixture, the solution was kept in a vacuum chamber to remove any air bubbles. Once a bubble-free PDMS solution was obtained, it was cast on a cleaned slide glass. Finally, the PDMS film was cured in an oven at 80°C for 48 hr.
We used zinc acetate dehydrate (Zn(CH3COO)2‧2H2O, 99.999%, 0.01 M) dissolved in 100 mL ethanol as a seed solution. The seed solution was dropped on the PDMS substrate and the seed layer was heated to 90°C for one hour. The ZnO seed coated PDMS film was then immersed in a zinc nitrate hexahydrate (Zn(NO3)2‧6H2O, 98%, 0.025 M), hexamethylenetetramine, (CH2)6N4, (HMTA, 0.025 M) and deionized water (200 mL) aqueous solution. After heating at 90°C for 3 hr, the grown film was rinsed with DI water and dried at room temperature. Finally, the ZnO-coated PDMS film was peeled off from the glass substrate for study.
The morphology of the aligned ZnO nanorods on PDMS was studied by scanning electron microscopy (SEM, Hitachi 3400S). X-ray diffraction (XRD) analysis was also carried out on the deposited ZnO nanorods (Rigaku Miniflex II instrument with Cu-Kα radiation, λ= 0.154 nm). A photoluminescence (PL) spectrum was run by (Picoquant Fluotime 200 spectrometer) at room temperature.
*Sylgard 184 silicon elastomer base, Dow Corning, Midland, Mich.
**Sylgard 184 silicon elastomer agent, Dow Corning, Midland, Mich.
Results and discussion
The ZnO nanorod array growth mechanism has been reported in the literature.15,16 Here, ZnO seeds were formed on the PDMS substrate first by the sol-gel heating method. For ZnO nanorod growth, the chemical reaction was presented as follows:
(CH2)6N4 +6H2O → 6HCHO + NH3 (1)
NH3 + H2O ↔ NH4+ + OH- (2)
2OH- + Zn+2 → Zn(OH)2 (3)
Zn(OH)2 → ZnO + H2O (4)
Zinc nitrate and HMTA were used as the source of Zn and OH-. When the zinc nitrate and HTMA solution is heated, the HMTA begins to decompose to NH3 as in Eq. (1). The ZnO seeds start to grow on the PDMS substrate as shown in Eqs. (3) and (4).
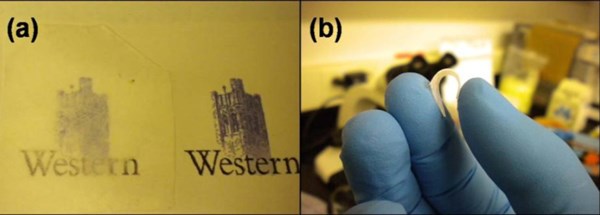
Figure 1 - (a) Optical microscopic image of the ZnO nanorod arrays coated on PDMS; (b) the flexible ZnO nanorod arrays coated on PDMS.
Figure 1(a) is the optical microscopic image of the ZnO nanorod arrays coated on a PDMS substrate. The sample of the ZnO nanorod-coated PDMS (left mark) has a relatively high light transmission. Further, the translucent sample has excellent flexibility as shown in Fig. 1(b). This hybrid material has potential use in flexible electronic devices with good light transmission.
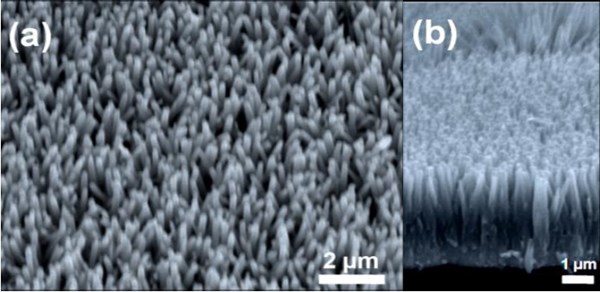
Figure 2 - (a) Top view SEM micrograph of ZnO nanorod arrays on PDMS; (b) cross-sectional SEM image of ZnO nanorod arrays.
Figure 2(a) shows a top view SEM micrograph of ZnO nanorod arrays on PDMS. The dense and uniform ZnO nanrods grow normal to the PDMS surface. The average diameter of the ZnO nanorods is estimated to be 161±10 nm. In addition, the cross-sectional SEM micrograph of ZnO nanorod arrays on PDMS is shown in Fig. 2(b). All nanorods are well-aligned and normal to the surface of the PDMS. The average height of the nanorods is ~2 µm.
The crystalline structure of the aligned ZnO nanorods was further studied by a scanned XRD powder profile. As shown in Fig. 3, ZnO wurtzite characteristic peaks can be seen as (100), (002), (101), (102), (110), (103) and (201).15,16 Compared with other peaks, the (002) diffraction peak has much stronger intensity, revealing that the nanorods were preferably aligned in the c-axis polar direction.17 The diameter of the ZnO nanorod was calculated using Scherrer’s formula:16,17
kλ
D = ——— (5)
where k is the shape factor of the crystalline (0.9), λ the x-ray wavelength (0.154 nm), B the full width at half maximum (FWHM) of the (002) diffraction peak, and θ the diffraction angle. The diameter calculated by Scherrer’s formula is 145.9 nm. The value is close to the results measured by SEM.
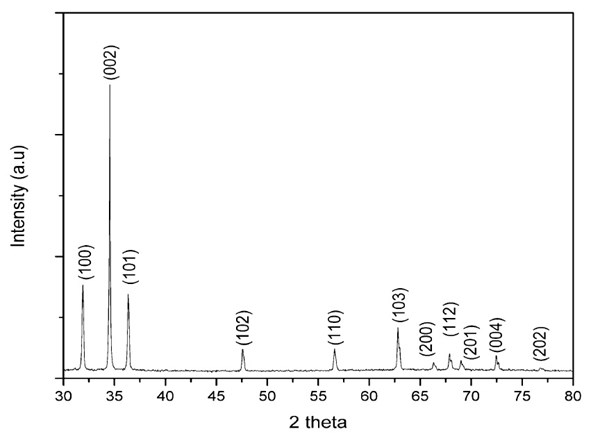
Figure 3 - XRD pattern of ZnO nanorod arrays on PDMS.
The room temperature photoluminescence (PL) spectrum with an excitation wavelength, λex, of 280 nm is shown in Fig. 4. ZnO nanorod arrays on PDMS have an emission, λemax of 390 nm. The broad emission peak may be attributed to the recombination of free excitations.18 It is well known that ZnO normally has three major peaks: a UV near-band-edge emission (NBE) peak around 390 nm, a green emission peak around 520 nm, and a red or orange emission around 600 nm, where the green and red/orange bands have been ascribed to the oxygen vacancies, interstitial zinc ions and impurities.18-20 The disappearance of these peaks indicates that the ZnO nanorod arrays have high purity with no defects.
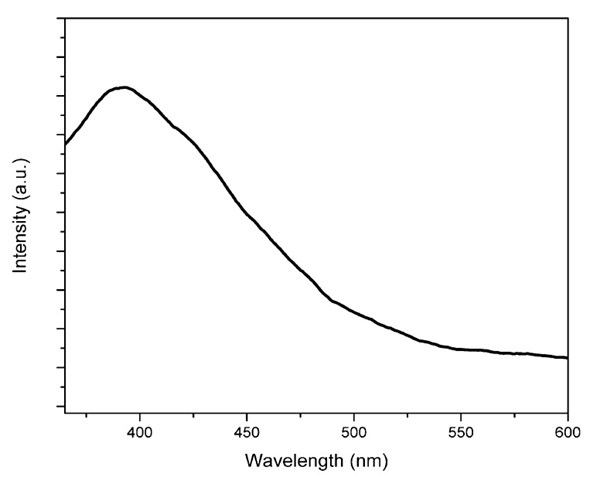
Figure 4 - Room temperature PL spectrum of ZnO nanorod arrays on PDMS.
Conclusions
In this work, we have presented a low temperature chemical synthesis method for ZnO nanorod arrays on flexible substrates. ZnO seeds were formed on PDMS substrates. Well-aligned ZnO nanorods with ~161 nm diameters were successfully deposited and along the normal of the surface of PDMS by a hydrothermal process. This c-axis direction-aligned ZnO nanorod shows a strong band emission peak located at 390 nm. The uniform and high purity ZnO coated films also show good flexibility and light transmission. Our results indicate that the ZnO nanorod arrays coated on PDMS have potential applications in flexible optical/bio devices.
Acknowledgement
This study was support by the Grant Challenges Canada-Canadian Rising Stars in Global Health, and the financial support from the University of Western Ontario (UWO).
References
1. S. Kumar, V. Gupta & K. Sreeivas, Nanotech., 16 (8), 1167 (2005).
2. Z. Zhang, et al., Talanta, 73 (3), 523 (2007).
3. N. Sekine, et al., Organic Electronics, 10 (8), 1473 (2009).
4. J.W. Rasmussen, et al., Expert. Opin. Drug Deliv., 7 (9), 1063 (2010).
5. E.A.A. Neel, et al., J. Mater. Sci: Mater. Med., 19 (4), 1669 (2008).
6. H. Karami, Int. J. Electrochem. Sci., 5 (5), 720 (2010).
7. Y. He, et al., J. Nanopart. Res., 7 (2-3), 307 (2005).
8. Q.R. Hu, et al., J. Alloy Compd., 496 (1-2), 494 (2010).
9. X. Zhou, et al., J. Cryst. Growth, 269 (2-4), 362 (2004).
10. M-Y. Choi, et al., Adv. Mater., 21 (21), 2185 (2009).
11. B. Baruwati, D.K. Kumar & S.V. Manorama, Sens. Actuat. B: Chem., 119 (2), 676 (2006).
12. Y. Xi, et al., Solid State Commun., 141 (9), 506 (2007).
13. X. Wang, C.J. Summers & Z.L. Wang, Nano Lett., 4 (3), 423 (2004).
14. Z. Yang, et al., Sens. Actuat. B: Chem., 135 (1), 57 (2008).
15. Z. Liu, et al., Appl. Surf. Sci., 255 (12), 6415 (2009).
16. R.S. Kumar, et al., J. Alloy Compd., 506 (1), 351 (2010).
17. D.K. Bhat, Nanoscale Res. Lett., 3 (1), 31 (2008).
18. L. Wang, et al., Appl. Phys. Lett., 86 (2), 024108 (2005).
19. D.C. Agarwal, et al., J. Phys. D: Appl. Phys. 42 (3), 035310 (2009).
20. E. De La Rosa, et al., J. Phys. Chem. C, 111 (24), 8489 (2007).

















