Ionic Liquids for Cleaning Operations at Air Force Air Logistics Centers: Part II
Appears in Print as: 'Testing Ionic Liquids for Cleaning Operations at Air Force Air Logistics Centers: Part II'
Researchers evaluate cleaners for potential use in wiping applications.
INTRODUCTION
Many industrial solvents used in cleaning operations have been designated by the United States Environmental Protection Agency (U.S. EPA) as producing greenhouse gases (GHGs) or containing volatile organic compounds (VOCs), hazardous air pollutants (HAPs), and/or ozone-depleting substances (ODSs). Because these chemicals lead to worker health and safety concerns, alternatives have been sought. In Part I of this paper published in Products Finishing on April 1, 2012, it was noted that ionic liquids (ILs) have been considered for cleaning applications because of their negligible vapor pressure (VP), high thermal and electrochemical stability, and low melting points (less than 212 degrees Fahrenheit (°F)) . Cation/anion pairs in ILs can be chosen to ensure an innocuous toxicology, and appropriate mass transport properties and reactivity with water of the IL can also be tailored by the choice of cation/anion pair , .
Featured Content
Because published work showed that 2-ethylhexyl lactate (2ehl) displayed similar performance to hydrofluorinated ether (HFE) 7100, which is currently used in vapor degreasing operations at Air Logistics Centers (ALCs), 2ehl was chosen for investigation as cleaning agent. . Although 2ehl is an organic solvent with low vapor pressure rather than a traditional IL, it has a considerably lower melting point in the mixed form than in each individual component, similar to traditional ILs. Based on chemistry and toxicology information, 1-ethyl-3-methylimidazolium (EMIM) acetate and EMIM ethylsulfate were also selected as being potentially good cleaning agents . Promising results were obtained for cleaning efficiency and materials compatibility testing1; therefore, chemical properties evaluations and hydrogen re-embrittlement testing of these cleaning agents were conducted on 4130 substrates.
EXPERIMENTAL DETAILS
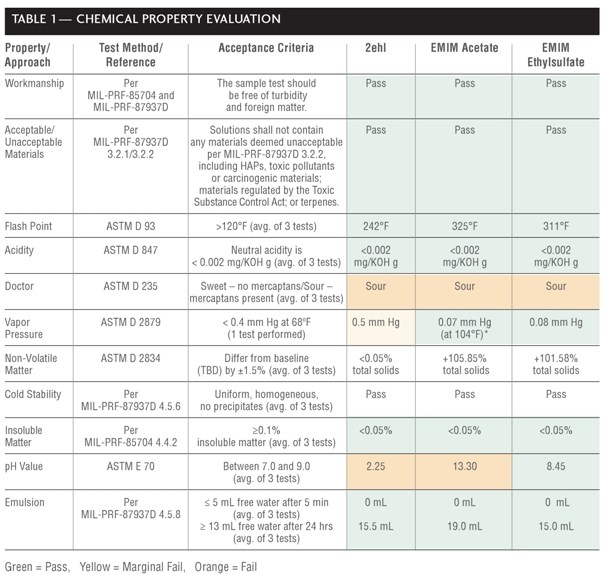
EMIM acetate, EMIM ethylsulfate, and 2ehl were procured from Government Scientific Source, Expotech USA, Inc., and Purac America, Inc., respectively. Testing was derived from performance requirements for cleaning operations, which were extracted from technical orders and military specifications that had been supplied by ALCs paint/depaint and plating facilities -15. Testing followed industry and federal standards, including ASTM, U.S. EPA standard, and ALC military specification test methods as shown in Table 1.
Chemical Evaluations
Chemical properties evaluations (Table 1) included workmanship, unacceptable materials contained within the cleaners, flashpoint, neutral acidity, pH, VP, cold stability, emulsion characteristics, presence of mercaptan sulfur (doctor test), and non-volatile matter (NVM) and insoluble matter content.
Workmanship was visually examined through a clear, glass 250 mL beaker containing approximately 250 mL of concentrated cleaner (see Figure 1). Each cleaner passed because it was free of turbidity and foreign matter and was homogeneous (no visible separation of undisturbed solutions).

Material safety data sheets and technical data sheets were reviewed to determine whether any cleaners possessed:
• Unacceptable materials identified in 40 CFR 261
• Toxic pollutants listed in 40 CFR 301
• HAPs listed in 40 CFR 63
• Known carcinogens (as defined by the National Toxicology Program)
• Abrasives or chromates
• Heavy metals [cadmium, lead, mercury (Hg)]
• Phenols, creosols, or ketones
• Chlorinated compounds
• Ozone depleting substances in detectable amounts
• Sodium chloride, urea, sodium sulfate, nitrites, nitrates
• Sucrose or any unacceptable sugars (other than required, active ingredients).

No tested agent contained unacceptable materials or fell into Type I cleaner category (e.g., containing 25-40% by weight terpene hydrocarbons). All tested agents fell into the Types II, III, and IV cleaner category (i.e., all grouped together), which were composed of surfactants, adjuvant solubilizers for greases and oils, alkaline builders, water conditioning agents and corrosion inhibitors).
For flashpoint testing, 75 milliliter (mL) of undiluted cleaner was placed in a brass container held initially at 32ºF and stirred at 250 ± 10 rotations per minute (min). A flame (diameter between 0.126 inch (in) and 0.189 in) then heated the sample at 3°F per min until ignition source caused a distinct flash in the interior of the test cup, which was recorded as the flash point. Each concentrated solution had a flashpoint greater than 120°F, thereby passing MIL-PRF-87937D.
Acidity testing was conducted by mixing 50 mL of concentrated cleaner with 50 mL of isopropyl alcohol and automatically titrating with 0.1N potassium hydroxide (KOH) using a Metrohm Titrando, Model No. 836 autotitrator until a potentiometric endpoint (denoted by pink color) was reached. The volume and normality of the KOH and cleaner density were used to calculate the endpoint (acidity) in milligram per KOH per gram (g) [mg/KOH/g]. All solutions demonstrated neutral acidity, using the potentiometric method in ASTM D 1613.
For the doctor test, 125 g sodium hydroxide was mixed in 1 liter (L) reagent water, and 60 g lead monoxide was added to the solution and shaken vigorously for 15 min. A 5 mL aliquot of this solution was mixed with 10 mL undiluted test solution, shaken vigorously for 15 seconds (s) in a capped test tube, and a few milligrams of pure, dry sulfur was added. The test tube was shaken for 15 s, allowed to settle for 2 min, and observations were recorded. All samples were discolored or the yellow color of the sulfur film was masked, indicating the presence of mercaptan sulfur. Interestingly, only EMIM ethyl sulfate has a thiol present in its structure. Therefore, it is thought that the colors of the test solutions might be interfering with the qualitative test.
For VP testing, an isoteniscope and manometer filled with test solution under nitrogen was subjected to de-pressurization and re-pressurization in a constant-temperature bath heated incrementally by 13.8°F up to 68°F. Only 2ehl displayed a VP slightly above 0.4 millimeter (mm) Hg, not quite meeting the requirement of MIL-PRF-680B, yet not unexpected because it is not strictly an ionic liquid. Note that EMIM acetate possessed a VP below the detectable limit of the laboratory equipment, when the test was conducted at 68°F; therefore, the test was attempted again at 104°F and still displayed an acceptable value that was below the requirement.
Non-volatile matter testing (NVM) was conducted for informational purposes. Pass/fail ratings were not assigned because no baseline has been chosen for comparison. NVM testing was performed by placing two grams of each agent on an aluminum dish and into an oven maintained between 216.5°F and 225.5°F for 4 hours (hrs). Specimens were weighed upon cooling to obtain the total solids weight. NVM was calculated by multiplying the solids weight by 100, dividing by the total sample weight (prior to heating), and averaging three tests. 2ehl had less than 0.05% NVM, while EMIM acetate and EMIM ethyl sulfate had greater than 100.0% NVM, which could be attributed to chemical composition changes due to reaction of the test solutions with air or changes in composition (or decomposition) at elevated temperatures.
In cold stability testing, 50 mL of each cleaner was cycled five times from room temperature to 32°F to ensure cleaners maintained their homogeneity and uniformity after cycling (i.e., conformance to military cleaning specifications). All three test solutions passed.
Two 100 g samples of each cleaner was vacuum (e.g., 200 to 250 mm Hg) filtered through a Whatmas No. 1 equivalent filter lined Buchner funnel for five minutes and was weighed to quantify the amount of insoluble matter. Each cleaner passed, having less than 0.05% insoluble matter.
An Orion Research Digital pH/millivolt Meter Model 611 pH meter was calibrated and used to measure the concentrated test solutions pH at room temperature. Only EMIM ethylsulfate passed, displaying a neutral pH (between 7.0 and 9.0) in the undiluted form. 2ehl was highly acidic (pH 2.25), while EMIM acetate was strongly basic (pH 13.30). It is suspected that the pH for 2ehl is inaccurate because it was likely that the pH probe had residual water on it (after cleaning). The presence of water on the pH probe may have altered the pH results of the non-aqueous test solutions. Specifically, 2ehl is insoluble in water, and additional pH testing using a pH probe that has been cleaned with a non-aqueous solvent is necessary for 2ehl. Furthermore, due to the non-aqueous structures of EMIM acetate and EMIM ethylsulfate, there is potential for the anions to interact with the water and increase the pH. This effect may be more responsive with EMIM acetate.
Emulsion characteristics were investigated by mixing 20 mL of lubricating oil with 25 mL of 1:3 cleaner:water dilution and shaking it vigorously for 15 s. 2ehl is insoluble in water; thus, it was expected for 2ehl to exhibit good emulsion characteristics with oil. All three cleaners had no free water existed after 5 min, and 15.5 mL (for 2-ehl), 19.0 mL (for EMIM acetate), and 15.0 mL (for EMIM ethylsulfate) of free water was observed after 24 hrs, thereby demonstrating good emulsion characteristics.
Because the cleaners passed most chemical properties evaluations and those tests that weren’t passed were questioned because the tests are not typically performed on these types of chemicals, the team determined that preliminary hydrogen re-embrittlement testing should be performed.
Hydrogen Re-embrittlement Testing
Hydrogen re-embrittlement testing was conducted per ASTM F 519 immersion, sustained-load, as a service environment test. Specimens were fully immersed in each cleaner for 155 hrs and loaded at 45% notched fracture strength (NFS) at temperatures between 68 and 86°F. The acceptance criterion stipulates that specimens shall not incur fractures. If one specimen fractures, the remaining three specimens must be subjected to 2 hr 5% incremental increase in stress to a maximum of 90% NFS. Concentrated EMIM ethyl sulfate was non-embrittling to 4340 steel, lasting 150 hrs at 173.8 kilopounds-force per square inch (ksi) applied stress. However, both concentrated and/or diluted forms (e.g., 1:1 ratios with water) of 2ehl and EMIM acetate could not be tested because both dissolved the Lexan test solution containers. The concentrated solutions leaked onto 4130 steel dummy bars used for the test set-up and remained there, unknowingly, for 24 hrs. Dummy bars were wiped clean, and test set up continued. During specimen loading, the dummy bars fractured, suggesting that the test solutions had embrittled the 4130 steel. Likewise, the test set up was trialed for each cleaner diluted with water at a 1:1 ratio. Again, both diluted cleaners dissolved the containers again. Testing was not further pursued with 2ehl or EMIM acetate.
CONCLUSIONS
The cleaners passed most chemical compatibility testing, except the doctor test, which indicated the presence of mercaptan sulfur in all three cleaners. Because only EMIM ethylsulfate has a thiol present in its structure, another quantitative test to detect sulfur content is recommended for follow-on activities. EMIM acetate and 2ehl also failed the neutral pH requirement (being strongly basic and acidic, respectively). Upon investigation, it was found that deionized water was used to clean the probes between pH measurements. Because the cleaners are non-aqueous, there was a concern that the results were affected by rinsing the probes. Testing should be repeated for each cleaner using optimized dilutions for obtaining maximum cleaning efficiency; dilution (with water or other solvent) will likely affect pH. Concentrated 2-ehl marginally failed the VP test. Because the cleaner will not be used in concentrated form, this test must be repeated in the optimized diluted form.
Modified hydrogen re-embrittlement testing using compatible test container materials and optimized dilutions, is also necessary to measure the embrittling nature of 2ehl or EMIM acetate. Additionally, axial fatigue testing will be required to determine effects of the cleaners on the integrity of sub-surface substrate properties.
Overall, the cleaners show potential ability for use in wiping applications, yet further evaluation is necessary to optimize cleaning performance, while ensuring material compatibility and conformance to military chemical compatibility requirements.
References:
1Berman, Elizabeth, Melissa Klingenberg, Natasha Voevodin, and Janelle Yerty, “Ionic Liquids for Cleaning Operations at Air Force Logistics Centers,” Products Finishing, April 2012.
2Abedin, Sherif Zein El, and Frank Endres, “Ionic Liquids: The Link to High-Temperature Molten Salts,” Accounts of Chemical Research, Nov. 2007.
3Abbott, Andrew P. and Katy J. McKenzie, “Application of Ionic Liquids to the Electrodeposition of Metals,” Phys. Chem. Chem. Phys., 2006, 8, 4265-4279.
4Rogers, R., et.al., “In Ionic Liquids as Green Solvents,” ACS Symposium Series, American Chemical Society; Washington, DC, 2003.
5National Aeronautics and Space Administration (NASA) Final Report and Deliverables, “Precision Cleaning of Oxygen Systems and Components,” NASA/CR-2009-214757.
6Dr. Markus Wagner, MERCK; Dr. Megan O’Meara and Dr. Aurelie Alemany, BASF Corporation; Dr. Khalid Shukri and Dr. Andrew Abbott, Scionix, telephone correspondence, November 2010.
7Technical Order TO 42C2-1-7, “Technical Manual: Process Instructions: Metal Treatments: Electrodeposition of Metals and Metal Surface Treatments to Meet Air Force Maintenance Requirements,” 28 Feb. 2006.
8Boeing Specification BAC-5408, “Vapor Degreasing, Revision M.”
9Boeing Specification BAC-5765, “Cleaning and Deoxidizing of Aluminum Alloys.”
10Boeing Specification BAC-5555, “Phosphoric Acid Anodizing of Aluminum for Structural Bonding.
11Military Specification MIL-PRF-87937, “Performance Specification, Cleaning Compound, Aerospace Equipment, 24 Sept. 2001.
12 Military Specification MIL-PRF-680, “Performance Specification, Degreasing Solvent,” 26 Oct. 2006.
13Technical Order TO 1-1-691, “Cleaning and Corrosion Prevention and Control, Aerospace Equipment and Non-aerospace equipment, 19 Oct. 2007.
14Technical Order TO 2-1-111, “Standard Maintenance Procedures, Navy and USAF, P&W Aircraft Engines.”
15Technical Order TO 1-1-8, “Application and Removal of Organic Coatings, Aerospace and Non-aerospace Equipment,” 12 Jan. 2010.
RELATED CONTENT
-
Zinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment
-
Top Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.
-
Preparation for Electroplating
What you should know about cleaning and electrocleaning.