Photovoltaic Progress
How electroplated silver can improve solar cell efficiency
#energy
When most people think of solar energy systems, they imagine photovoltaic (PV) panels like those found on solar-powered calculators or satellites. PV cells convert light directly into electricity, enabling them to power a tremendous number of modern electric and electronic devices.
The vast majority of PV solar cells are produced by screen-printing silver paste onto the front side of a silicon wafer, then firing to form the front-side contact.
Featured Content
Standard silver pastes contain many components, some of which are needed for application but are not beneficial for conductivity. Pure silver metal has resistivity of 1.59 microhm-cm at 20°C. Most silver pastes have double or triple that resistivity.
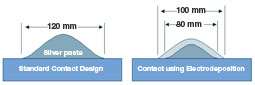
In addition, silver pastes flow when they are fired and thus are limited in the cell aspect ratio that can be achieved. As a result, most manufacturers print a 120-µm wide line for the front side contact to achieve a cross sectional area large enough to carry the current generated by the cell.
The silver paste process also introduces much of the variability experienced in cell efficiency. Addition of electrodeposited silver to the cell can improve the front side contacts, reduce product variability and increase cell efficiency by up to 0.4% absolute.
It may sound small, but this type of improvement has captured the interest of many solar cell manufacturers. Still, many producers are hesitant to adopt electroplating because cell efficiency gains depend on many factors that can be unique to an individual cell.
Electro-Experiment
In an attempt to isolate the effects of the electrodeposition process on cell efficiency and consistency, our company worked with solar cell manufacturer Evergreen Solar Inc. (Marlboro, MA) to manufacture a batch of 156 cells using electrodeposited silver. The electrical characteristics of these cells were measured both before and after electrodeposition onto the front side contacts. Efficiencies were measured, but the focus was on the change in front side resistance (Rfront) resulting from electrodeposition.
Test cells were manufactured using a standard process flow and then tested for efficiency and Rfront. They were processed through a light-induced plating (LIP) tool, where light was introduced to the cell to generate some of the power needed for the electrodeposition process. A rectifier was used to put a voltage potential on the backside of the cell to protect the backside contact from becoming the anode and dissolving during the electrochemical reaction.
Electroplated in Technic, Inc.'s TechniSol Ag silver plating bath for 10 min at room temperature resulted in 8-10 µm of fine-grain plated silver being deposited on top of the silver paste front side contacts. Plating current density was 1.3 A/dm2.
Subsequent testing has shown this thickness of electrodeposited silver can be achieved in 5 min if solution temperature is raised to 40°C and agitation is increased. This allows plating of a fine-grain silver deposit at 2.6 A/dm2.
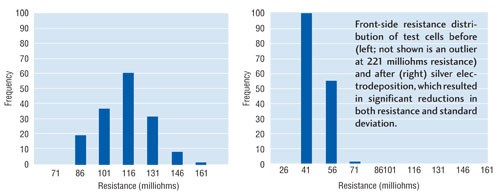
Electrical characteristics of cells were retested after electro-deposition. Results showed that depositing silver metal onto the silver paste contacts dramatically reduced average Rfront. Before silver electrodeposition, the batch of test cells averaged Rfront of 122 milliohms. After plating process, average Rfront was 54 milliohms.
Perhaps more significant was the effect on data distribution. Cells in this experiment started with a standard deviation of 18 milliohms before plating. After plating, the standard deviation had dropped to 6 milliohms. So, not only did the overall resistance drop, variability in Rfront was significantly reduced. The effect was most pronounced on those cells with a relatively high initial resistance.
The final steps for the test cells were assembly into a module and reliability testing, which consisted of exposure to 1,000 hr of damp heat conditioning (85°C at 85% relative humidity). Retested after exposure, modules using the electroplated cells experienced less than the allowable 5% degradation in power. In fact, the test module's power dropped by only 1% over the test.
Application Approaches
There are two ways cell manufacturers can use electrodeposition to improve their product. One requires a wholesale change in cell design; the other uses electro-deposition as a remedial process to improve cells with low efficiency due to high resistance.
The design change approach takes advantage of the lower resistance of electrodeposited silver to allow reduction in silver paste line width from 120 to 80 µm. At 120 µm wide, the resulting grid pattern shades approximately 7% of the front of the cell. Reducing line width to 80 µm and adding 10 µm of electrodeposited silver allows more light to enter the cell and translates directly into higher power output. Cell efficiencies increased by 0.3-0.5% absolute depending on cell design and processing.
Like any added processing step, addition of silver electro-deposition as a remedial process increases manufacturing costs. However, results of this study open a possible path for manufacturers who are hesitant to add this cost to their entire product line.
As pointed out earlier, efficiency gains from electrodeposited silver were highest for cells that saw the greatest reduction in Rfront. Even without a change in contact design, cells that experienced a decrease in Rfront of 80-120 milliohms demonstrated an absolute efficiency increase of 0.25-0.50%.
It is possible to predict the change in Rfront that the electro-deposition process will induce by simply measuring Rfront after firing. This measurement does not require full characterization of the cells' electrical properties. In fact, a simple in-line resistance tester could be used to identify cells with a front side resistance above a certain threshold. Those cells could then be passed through the electrodeposition process to overcome the condition that is creating the increased resistance.
Reducing Cost
Electrodeposition is different than other processes used in most solar cell production lines, and often enough there is little or no electroplating experience within the manufacturer's technical staff. Keeping this in mind, there are a few aspects of the electroplating solution to be considered.
Perhaps the most important is the cost of the plating solution. More silver is removed from the process as drag-out than on wafer surfaces leaving the plating tank. To minimize losses due to drag-out, silver concentration in the solution should be kept to a minimum—ideally, 20 g/L or less of silver metal. In addition, a silver recovery system should be installed on the rinse chambers of the electrodeposition tool to recover as much of the lost metal as possible.
There are indirect costs to consider as well. To reduce overhead, a good silver electrodeposition process should be easy to analyze and control, stable and adjustable to the customer's requirements. Mistakes in electroplating process control can result in the loss of precious metals, so great care needs to be taken to ensure that responsible employees are trained by the solution supplier.
Acknowledgement: The author would like to thank Evergreen Solar Inc. for their assistance in this study.
RELATED CONTENT
-
Choosing and Troubleshooting Copper Electroplating Processes
Learn more on this inexpensive and highly efficient process.
-
Smut and Desmutting
Question: I am new to this industry and have heard about smut and desmutting operations.
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.