Plate Better, Longer
Electrolyte maintenance techniques can extend bath life
#pollutioncontrol
Electroplating baths are subject to constant changes in composition caused by the plating process itself, by water and impurities which are dragged into the solutions, and by electrolyte drag-out. This list doesn’t even mention other possible factors, such as evaporation, anodic and cathodic ancillary reactions, false dosing, and absorption of components from the air (for example, absorption of carbon dioxide in alkaline baths, dust, or micro-organisms).
All this means that the electrolyte must be regenerated either constantly or at intervals to avoid the eventual need for a new bath make-up and disposal of the spent solution. Several regeneration processes are available.
Featured Content
Particle extraction such as metallic flitter, dust, colloids, micro-organisms, various metal compounds and anode sludge is without doubt the first and most important method of bath maintenance. Without filtering, plated deposits would be rough. The majority of filters can be operated with filter aids such as celite or active carbon (with back-washing). However, this type of filtration cannot be performed while the electrolyte is working.
Another process which has proved itself under practical working conditions is UV-H2O2 oxidation. In this case part of the bath volume of (for example) a bright nickel bath is treated with UV/H2O2. Oxidation is performed at intervals outside the tank, and breaks down all organic compounds and decomposition products as well as the effective brightening additives and wetting agents. This treatment leads to a Watt’s Basic make-up, which can be mixed into the bath again; however, this technology does not work in a bypass system, and the UV-H2O2 units are quite expensive.
This article will discuss two technologies—one new and one improved—for electrolyte maintenance that can perform in bypass systems: adsorber polymer technology and liquid-liquid extraction.
ADSORBER POLYMERS
Adsorber polymers can be regenerated and can handle bypass operation. Divinyl benzol co-polymer resins, which belong to no functional group and thereby have no ion-exchange function, have proved themselves in this respect. Polarity, and more specifically the inner surface areas as well as the diameters of pores, can be determined by the resin manufacturing technique used.
Adsorber polymers can remove most of the organic components currently used in plating baths as well as the accompanying decomposition products. Operating conditions such as pH, the strength of the ions, the type and concentration of the organics used, and the flow-rate in the adsorber columns all affect the efficiency of the adsorber polymers, and not all resins can be used for every application. Laboratory results have shown that in several cases combinations of two or more polymers show the best performance in electrolyte purification.
The adsorber polymer must be regenerated after bath purification by an oxidation with 1-3% strong H2O2 by desorption of the adsorbate with 3-6% sodium lye. Surplus H2O2 must not be allowed to enter the wastewater treatment plant and, because the sodium lye and the alkaline rinse water contain all organic substances used, total organic carbon (TOC) concentration of the wastewater increases. An acid post-treatment balances pH. Both the oxidative pre-treatment and the sodium lye used will gradually attack the resin, necessitating replacement roughly every two years.
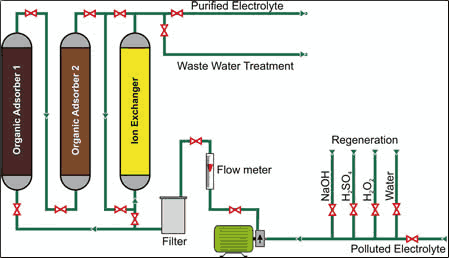
Other contaminants such as metal ions also can be stabilized by ion exchange, but until recently the regeneration procedure had to be customized for each application. One way to address this issue is using a universal adsorber polymer unit consisting of several columns filled with different polymers and ion exchangers that can purify various plating electrolytes. The adsorber columns can be regenerated separately to optimize performance for each. A single treatment with such a system can reduce unwanted drag-ins and breakdown products by up to 50% depending on the size of the unit and the quantity of impurities in the bath. The reduction depends on run time.
Eventually, the adsorber columns become saturated with organics and lose cleaning performance. In laboratory tests, after 200 minutes the columns released some impurities in a higher concentration than that in the cleaned plating tank. The reason for this is the chromatography effect: adsorption is not static; it is mobile within the column and impurities are located throughout the column. This results in a high outgoing impurity concentration. When this occurs, the adsorber treatment must be stopped and the resins regenerated.
Besides removal of “bad organics,” adsorber polymers can also remove significant amounts of “good organics” such as brighteners. These materials can be replenished by recharging the adsorber polymer. Also, as mentioned previously, inorganic contaminants can be removed by adding ion exchange to the adsorber polymer. Such a system can easily reduce Zn2+, Cr3+, Al3+, Cu2+, Fe2+/3+ and other impurities by up to 60%. This unit has been developed and marketed by Enthone Inc. as the EnPure nickel purification system.
LIQUID-LIQUID EXTRACTION
As the name implies, liquid-liquid extraction removes impurities from a carrier liquid—in this case, the electrolyte to be cleaned—with the help of a liquid extraction medium. Movement of contaminants from one liquid to another results from the different solubility of the two liquids, and their different concentrations. Extraction medium and carrier liquid should be insoluble to ensure good separation and minimize solvent drag-out. Generally, one of the liquids will be aqueous, the other an organic solvent, that is, the solution from the extraction medium in an organic solvent.
The solvent must have the following characteristics:
- High solubility of the material to be extracted
- High selectivity
- Large density difference to the carrier liquid, to ensure good separation
- Must not prevent emulsification
- Good regeneration characteristics
- Favorable price.
In liquid-liquid extraction, contaminants are distributed between two media that cannot mix with each other. For this reason replacement and the establishment of equilibrium takes place via the phase interface. A high phase interface will accelerate the establishment of equilibrium.
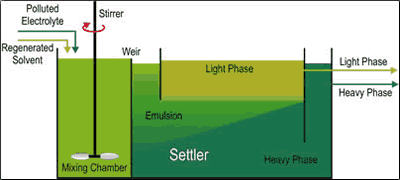
Large technical extraction applications use the mixer-settler principle. The extraction medium and the carrier liquid mix with each other in the first chamber (mixer), then go to the so-called settler over a weir. Here the phases can separate again, and can then be removed separately from the chamber. Several such units can be connected in sequence to achieve the desired cleaning results.
A prerequisite for the successful use of liquid-liquid extraction in electroplating systems is to identify a solvent that: a) selectively dissolves impurities; b) is easy to separate; and c) leaves very little residue in the bath so that no plating faults occur. Acetic acid butyl ester has proved to be a likely candidate to meet these requirements. Laboratory experiments showed this compound could remove impurities from bright nickel electrolytes to such an extent that they were no longer detectable by HPLC, while leaving additives such as brighteners in the bath.
Tests with the regenerated electrolytes also showed that the extraction medium had no negative affect on plating deposits. However, they had to remain in the settler for an extended time: in some cases, it took several minutes until the aqueous, salt electrolyte phase had separated itself from the butyl acetate phase. Here the decisive factor is the effect of the surfactant charge which sometimes generates very stable emulsions.
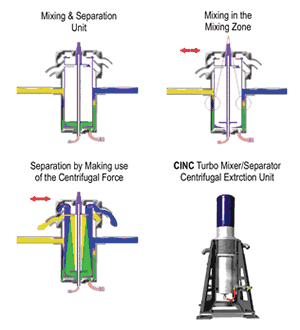
Continuous bath cleaning using liquid-liquid extraction as described thus far requires use of centrifugal extractors, which mix the carrier fluid and the extraction medium by shearing and then separate them again using centrifugal force and taking advantage of the density difference between the solvent and the electrolyte. During processing, the emulsion with the higher density will be thrown outward by the rotation action while the part with the lower density remains in the center of the separator. A density-ratio-specific weir can then separate the two phases. Such a system lowered concentration of a variety of impurities by 60-90% while leaving important additives such as brighteners in the bath.
Optimal process parameters were a low rotation frequency while mixing and short exposure time (six sec). Short exposure time with good extraction results mean high throughput quantities, and low rotation frequencies mean less wear on the extraction unit. Using a row of centrifugal extraction units may provide even higher efficiency and make possible low-cost bypass operation with no loss of efficiency.
DIAPHRAGM-SUPPORTED EXTRACTION
The liquid-liquid extraction processes described above are based on formation of an emulsion followed by phase separation, which can be problematic. A different approach is diaphragm-supported liquid-liquid extraction, in which the organic extraction medium and the carrier (electrolyte) are separated by a microporous diaphragm.
In this technique, the organic phase will spontaneously moisten the diaphragm and endeavor to migrate to the other side through its pores. A slight over-pressure can avoid such penetration on the electrolyte phase and fix the interface point between the aqueous and the organic phases in the mouth of the pores, where the driving force for material exchange becomes the concentration gradient. The liquids are simply led past both sides of the diaphragm.
Users of this technique can adjust the flow rate of both phases over a wide range without fear of any penetration problems. Systems with a tendency to form emulsions lend themselves to the approach. Unlike the systems described above, no moving particles will be found in the extraction unit during diaphragm-supported liquid-liquid extraction.
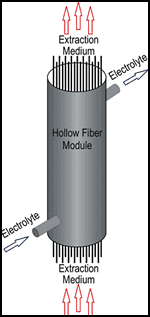
The process can also be implemented using so-called hollow fiber modules. These consist of a large number of porous capillaries through which extraction medium and electrolyte flow.
In laboratory tests carried out with hollow fiber capillaries with an inner diameter of about 1.8 mm, wall strength of 0.45 mm and a microscopically small pore diameter of about 200 µm, cleaning depended on flow rate of both the extraction medium and the electrolyte as well as time. As might be expected, longer electrolyte dwell time in the system and higher flow rate of the extraction medium—in this case, butyl acetate—resulted in improved cleaning.
In testing, an extraction medium flow rate of 2.0 liter/min and dwell time of 90 sec or more resulted in a 95% reduction in all types of contaminants. Even the worst laboratory operating parameters resulted in a 20-50% in measured contaminants. With bypass operation, this system could considerably increase the operational life of an electroplating bath; however, practical use is complicated by the odor and the low ignition point of the butyl acetate extraction medium.
CONCLUSIONS
Adsorber polymer technology is user-friendly and safe, and is already in use in several plating facilities. Because it requires periodic regeneration of the adsorber polymer, it can remove contaminants from plating baths in what is best described as a “semi-bypass” process. A well-chosen adsorber system can significantly reduce bath contamination and thus improve bath performance and stability. Two points that must be addressed are the initial capital investment and the relatively high cost of replenishment.
Liquid-liquid extraction shows significantly higher selectivity than the adsorber polymer technique, efficiently removing contaminants while leaving needed additives such as saccharine brightener in the bath. The process can be operated in bypass mode to considerably increase bath operational life. However, other problems, including the relatively long time needed for phase separation in units based on the mixer settler principle, may occur. The most efficient extraction medium tested was butyl acetate, which has exhaust and odor concerns.
Diaphragm-supported liquid-liquid extraction using microporous pipes to allow the exchange of impurities without mixing addresses the phase separation issue by maintaining the phases separately. However, pressure must be kept stable on both sides of the membranes to avoid possible breakthroughs.
The author would like to acknowledge the work of several coauthors: his Enthone GmbH colleague C. Werner; A. Koenig of Friedrich Alexander University (Nuremberg, Germany); and B. Griffith and S. Peto of Enthone Inc. (West Haven, CT).
RELATED CONTENT
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.
-
Choosing and Troubleshooting Copper Electroplating Processes
Learn more on this inexpensive and highly efficient process.