PVD Coatings for Medical Device Applications
PVD is widely used for its wear resistant thin-film coating...
#medical #vacuum-vapor
Since it was introduced to the medical device industry in the late 1980s, physical vapor deposition (PVD) has become widely used to deposit wear-resistant thin-film coatings on a variety of medical devices, including orthopedic implants, pacemakers, surgical instruments, orthodontic appliances and dental instruments. The value of PVD technology rests in its ability to modify the surface properties of a device without changing the underlying material properties and biomechanical functionality.
PVD Coating Properties and Medical Devices
PVD coatings provide a number of benefits to medical devices in addition to hardness and adhesion. The most significant are listed in Table I. The biocompatibility of a coating is a prerequisite to its use on medical devices. For this reason, Multi-Arc has tested the biocompatibility of all the coatings that it applies, with the exception of TiN, which has been widely reported to be biocompatible.
Featured Content
Coatings are certified biocompatible based on a series of tests conducted by an independent biological-testing laboratory. These tests were conducted in accordance with ISO 10993-1 guidelines for materials that experience short-term body contact. The results indicate that TiN, ZrN, CrN, TiAlN, AlTiN and two Multi-Arc proprietary coatings (Blackbond and Tetrabond) are acceptable for external and internal medical devices that contact bone, skin tissue or blood.
Biocompatibility tests completed include the following:
- Sensitization: no significant evidence of causing delayed dermal contact sensitization in a guinea pig.
- Cytotoxicity: no evidence of causing cell lysis or toxicity.
- Acute Systemic: no significant systemic toxicity or mortality.
- Intracutaneous: no evidence of significant irritation or toxicity in rabbits.
- Genotoxicity: not mutagenic.
- USP Muscle: non-irritating to muscle tissue.
- Hemolysis: nonhemolytic, compatible with blood.
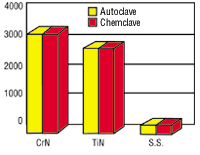
PVD coatings on medical devices must also be compatible with the sterilization process. Both TiN and CrN resist corrosion that can be caused by steam and chemical autoclaving.
| TABLE I—Ion Bond Coating Provides... Improved wear resistance Reduced friction Biocompatibility Decorative colors & aesthetic Chemical barrier Nickel Sensitivity Saline Solutions Sterilization
|
A multi-component assembly with both 416 and 304 stainless steel components experienced corrosion after autoclaving. The manufacturer tried to solve the problem by using a new grade of stainless steel but failed. TiN and CrN were autoclaved in deionized water at 132F and 30 psi and were chemclaved in vapor phase alcohol. The results showed that both coatings protected the underlying stainless steel and resisted autoclave-induced corrosion.
Orthopedic Implant Applications
TiN has been in clinical use on orthopedic implants for more than 9 years in North America and Europe. The most common applications involve total joint replacement with Co-Cr-Mo or Ti-6-4 alloy implants. TiN is used for hip, knee, shoulder and ankle implants.
Pappas and Buechel1 have reported that TiN coated Ti-6-4 femoral components outperformed uncoated Co-Cr-Mo in a 4-station hip simulator in DI water at 37C with a load oscillating from 0-2200N. The TiN coating tested was deposited with the Ion Bond process to a thickness of 9 µm. Components were polished before and after coating to obtain a surface finish of 0.04 µm Ra.
Results showed that a TiN coated Ti-6-4 resurfacing cup generated less than half the wear of an uncoated Co-Cr-Mo component after 10 million cycles. This is consistent with performance reported by Coil2. Coil and his coworkers attributed the reduced wear to the reduced adhesion of polyethylene to the surface of the implant. With uncoated titanium alloy implants, this can result in adhesive wear that actually rips pieces of titanium from the implant surface. These then oxidize to form third body TiO2 particles that can rapidly abrade polyethylene. TiN prevents this cascading failure mechanism from beginning.
| TiN - titanium nitride ZrN - zirconium nitride CrN - chromium nitride TiAlN - titanium aluminum nitride AlTiN - aluminum titanium nitride |
Moving components (hips, ankles, etc.) are also susceptible to abrasion by third body particles of bone or bone cement. In the case of an artificial knee, the particles are trapped between the metal femoral component and the polyethylene tibial component. The abrasive particles can cause damage to both surfaces. Scratches to the femoral component are the most detrimental to the long-term viability of an implant because they accelerate the wear of polyethylene
| TABLE II—Pin-On-Disc Abrasion Performance | ||
| Coating/Substrate TiN BlackBondTM (Me-CH) TetraBond® (Amorphous C) |
at 1 Million Cycles Measurable wear Worn through No visible wear |
at 5 Million Cycles Worn through Worn through No visible wear |
TiN is still the only coating used clinically on orthopedic implants, but AlTiN, BlackbondTM and Tetrabond® coatings are being tested as an alternative. The primary areas of interest are for hip and knee prosthesis, plates, and screws for fractures and spinal fusions. The abrasion resistance of thin films was tested, and the results are summarized in Table II.
The abrasion resistance of Tetrabond is attributed to its high hardness. The results of a test at Cambridge University3, which abraded it with 4 micron SiC, indicated that it is 100 times more abrasion resistant than TiN.
Fretting corrosion is another implant problem that is being addressed with PVD coatings. Fretting corrosion occurs when two metal components are subjected to micro-movement in the range of <250 µm in the presence of corrosive bodily fluids. The micro-movement removes the passive oxide film on stainless steel and titanium components, leading to corrosion and ion release from implants. In severe cases, large quantities of oxidized metallic wear debris are generated.
Titanium is particularly susceptible to fretting corrosion. This, coupled with the common use of titanium alloys for spinal implants, has led to concern regarding the long-term effects of these implants. Black wear debris in proximity to spinal implants has been reported in the clinical literature. Japan has reportedly disallowed the import of Ti-6-4 spinal implants.
As early as 1991, Mauer4 reported that TiN coating could reduce the fretting wear rate of Ti-6-4 screws and plates. The results showed the TiN coating significantly reduced wear on both the screws and the plates when the screws were coated with TiN. When both components were coated, the wear was even further reduced. These tests were conducted using a standard ASTM test F897 in which the fixtured screws and plates were immersed in 20 ml of 10% calf serum in 0.9% saline solution at 56C with micro-movement of 0-100 µm. In addition to measuring weight loss, metal ion levels in the solution were measured. Metal ion concentrations for the various surface treatments relative to uncoated Ti-6-4 were consistent with the weight loss measurements. Abrasion resistance was tested, and the results are in Figures 1 and 3.
Due to their corrosion properties, interest in PVD coatings has now spread from orthopedics to other medial specialties where TiN, ZrN and CrN are being evaluated as corrosion resistant coatings on other implants.
Surgical Instrument Testing
Our company's initial application focus was orthopedic implants and surgical instruments with a strategy to promote TiN. It became clear; however, within 2-3 years additional coatings would open new application niches. For example, orthopedic manufacturers wanted a coating with more abrasion resistance than TiN for their implants. Ophthalmic, arthroscopic and endoscopic instrument companies wanted a coating with lower reflectance than TiN. Other instrument manufacturers wanted multiple coatings to color-code their instruments. Yet, others wanted a coating that was metallic in color.
Our earliest instrument development was with Storz Instrument Co., a manufacturer of ophthalmic ENT and plastic surgery instruments. It was launching a new line of neurosurgical instruments and wanted to differentiate its product from those of its competitors. Its neurosurgical line was handcrafted in Germany from 400 series stainless steel.
| TABLE III—Reamer Tip Temperature (F) | ||
| Hole # 1 2 3 4 5 |
Uncoated 168.8 184.4 190.9 189.5 194.9 |
TiN Coated 114.9 131.1 146.6 153.6 154.2 |
Stainless steel alloys have traditionally been used for surgical instruments because they provide good corrosion resistance and hardness. When these instruments cut bone or other dense tissue, however, the material wears quickly. Although worn cutting edges can be resharpened, this often entails shipping the tools back to the manufacturer. Consequently, hospitals and surgical centers are obliged to stock multiple sets of instruments, an additional operating cost that is passed on to the patient.
Storz had a dual goal for differentiating its instruments: improved performance and distinctive appearance. To attain its goal, the company turned to Multi-Arc, which recommended TiN coating. Storz agreed that the decorative appearance of TiN offered an effective means to a distinctive looking product. It was unsure that the performance improvements reported for metal-cutting tools could be achieved in surgical instruments, however. To determine whether TiN could extend the life of its products, Storz tested theperformance of uncoated and coated scissors made of 420 stainless steel. In the test, scissors were automatically opened and closed repeatedly to simulate use in surgery, with cutting edges periodically checked for wear and rounding. Results showed that the uncoated scissors made an average of 12,000 cuts before the edges required resharpening, whereas the coated scissors averaged 100,000 cuts before requiring resharpening. This was an increase of 800%.
To corroborate the laboratory results, Storz delivered both uncoated and coated prototypes to hospitals for comparative evaluation. Hospital reports confirmed that the coated instruments retained a sharp cutting edge longer than did the uncoated instruments.
Testing of TiN by other instrument manufacturers has shown it to extend the life of other medical products. Tests conducted at Othy Inc. showed that TiN coated reamers wore less and cut more quickly when reaming predrilled holes in an artificial bone. The average time required to ream a hole with a depth of cut 1 mm decreased 36 - 59% with TiN, depending on the reamer size. The faster cutting performance is attributed to the high lubricity of TiN that results in reduced friction between the reamer and artificial bone. The material removal rate increased up to 127% with TiN.
Coated reamer tips also generated less heat. The temperature at the tip of the reamer after each cut is summarized in Table III for the 14-mm reamer.
The lower tip temperature of TiN coated reamers was attributed to better chip flow. TiN-coated reamers produced continuous string type chips that exited from the top of the flutes. Uncoated reamers produced microfine chips that tended to pack into the flutes rather than exit. The reduction of cutting temperature is a benefit because high temperature can cause death of the bone cells, which lengthens the time required for healing.
| TABLE IV—Intramedullary Reamer Cutting Performance (Time to ream a 1.4 inch deep hole, Depth of Cut - 1 mm) | ||
| Reamer Size 12 mm 13 mm 14 mm 15 mm 16 mm |
TiN Uncoated 13 sec 11 sec 9 sec 10 sec 10 sec |
Uncoated 20 sec 25 sec 22 sec 21 sec 20 sec |
Similar results were recently reported for Tetrabond coated cranial burrs for drills. Both fluted carbide and diamond abrasive burrs were coated with Tetrabond. Holes were cut in artificial bone at a constant rpm and load. The coated burrs had an average cutting temperature of 270F compared to 583F for uncoated. An even greater difference in cutting temperature was observed with coated diamond abrasive burrs. Coated burrs also had less buildup and cleaned more easily. (See Tables III and IV)
Other Applications
Although the initial marketing strategy was to pursue orthopedic implant applications and surgical instruments, other applications have developed as a result of customer inquiries. Two application segments of note are dental instruments and orthodontic devices.
The number of medial grade coatings and applications for them has increased dramatically since Multi-Arc first established its medical program in 1990. The investment in coating development, biocompatibility testing and application development is expected to drive continued growth in this profitable market segment for years to come.
RELATED CONTENT
-
Automation Smoothes the Way for Surgical Coatings
Medical device coating company discusses how automation enables increased abrasion resistance and improved insulative properties for electrosurgery.
-
Nanostructure of the Anodic and Nanomaterials Sol-Gel Based Materials Application: Advances in Surface Engineering
Porous alumina can be fabricated electrochemically through anodic oxidation of aluminum. This paper reviews sol-gel chemistry and applications, which also offers unusual nanoporous microstructures. The ability to control pore chemistry at different scales and geometries, provides excellent bioactivity, enabling the entrapment of biologically active molecules and their controllable release for therapeutic and medical applications.
-
The Need for Metal Finishing in the Medical Device Industry
In the medical industry, devices are used both internally and externally, so biocompatibility is critical.