Rethink Your Cleaning Process
Modern vapor degreasing systems are environmentally friendly, save energy
#energy #pollutioncontrol #vacuum-vapor
It’s January, and with the New Year comes a new budget. At many companies the emphasis has been and continues to be on cutting operating costs. There’s one potentially large source of savings right under your nose, if you’ll consider ditching your old aqueous cleaning system for a small, fast and efficient vapor degreaser. Today, many innovative manufacturers are doing just that, simply to save money on their electric bills.
Aqueous cleaning normally delivers quality results, but aqueous systems tend to be large and complex. Vapor degreasers are usually smaller and simpler, and that simplicity enables them to deliver a more consistent and headache-free cleaning process than many aqueous systems. In fact, a properly designed and maintained vapor degreaser can be more budget-friendly, parts-friendly and planet-friendly than an aqueous cleaning system of comparable capacity.
Featured Content
Low-Boiling Solvents
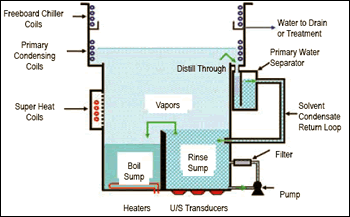
Many younger engineers today have never seen a vapor degreaser, so it might be worthwhile to review the technology. First, the term vapor degreasing describes a type of cleaning system based on solvents with boiling points of 90–170°F. Unlike water, these solvents also have low viscosities, low surface tension ratings, high densities, low specific heat and low latent heat. Used in properly configured equipment, they can deliver affordable, fast, reliable, safe and environmentally acceptable cleaning.
To begin cleaning, solvent is placed in the machine’s boil sump and heated using electric heating elements, hot water coils, steam coils or a heat pump unit. The boiling solvent produces a clear, dense vapor that rises into the chamber above and gradually displaces air there. This is the vapor blanket, and it helps contain the liquid solvent inside the machine.
Eventually, the vapors rise to the first set of refrigeration coils—the primary condensing coils—which chill them and condense the solvent back to its liquid state. This condensate drips into a trough that wraps around the internal circumference of the machine. The solvent then moves through a water separator, because some airborne humidity from above the vapor blanket also condenses.
At this point, the distilled solvent is routed into the rinse sump. Because the rinse sump already is filled with clean solvent, the addition of more solvent causes it to overflow and spill solvent back into the boil sump. This purging maintains a constantly clean rinse sump. It also concentrates any contaminants into the boil sump for easy removal and maintenance.
The 411 on H2O
How does all this differ from cleaning with water? Water may be the elixir of life, but when it comes to industrial cleaning there are better solvents. To overcome some of water’s natural chemical limitations, aqueous cleaning processes need more complex systems or use additives to make it a passable cleaning agent.
One issue stems from surface tension—the measure of the wettability of a liquid. The lower a liquid’s surface tension, the more easily it will flow across a substrate as well as around and under parts to be cleaned, creating more opportunities for cleaning to occur. In general, high surface tension equates to poor cleaning: If you can’t wet, you can’t clean.
Water has the highest surface tension of any popular cleaning agent, necessitating addition of surfactants to lower surface tension of aqueous cleaning formulations. Other additives such as detergents (alkaline formulations are common) are also used to boost the cleaning power of the water mixture, especially to help remove organic contaminants.
Even with these additives, the surface tension will still be higher than that of any modern solvent. Indeed, surfactants and detergents can actually be considered contaminants since it is sometimes difficult for the rinse water to remove them completely.
The second troublesome factor is viscosity. Water may seem thin compared with, for example, motor oil, but in comparison with modern solvents, water is the molasses of cleaning agents. A thick fluid like water resists flowing into tiny crevices or apertures and so is a less effective cleaning agent than thinner solvents.
This manifests itself with occasional “spotting” on cleaned parts. Additives and high-pressure sprays can improve system performance, but in general, a solvent with low viscosity is going to out-clean water simply because the low-viscosity material can get into and out of places that water cannot.
The third problem area with water is its density. When it comes to cleaning, heavier is better: many applications feature insoluble particulates that must be removed. A solvent with low surface tension and low viscosity, combined with high density, can more easily remove particulates from substrates and keep them suspended in the solvent. From there, it is a simple for a properly-designed filtration system to remove the particulate from the solvent.
In either type of system, cleaning usually is enhanced if the solvent is warm. The energy required to raise the temperature of a liquid is called its specific heat. It takes a lot of energy to raise the temperature of water, but low-boiling solvents have a lower specific heat and work at lower temperatures, which reduces energy consumption.
A related issue pertains to drying the parts after cleaning. The latent heat of vaporization measures the energy required to shift a chemical from liquid to gas phase. Aqueous cleaning systems will use more energy both to heat the cleaning agent above the boiling point of water and to dry water from cleaned parts than a system that uses a solvent with a lower latent heat of vaporization, lower specific heat and a lower boiling point. Again, modifications such as air knives are available for aqueous systems, but these increase energy consumption.
By the Numbers
Given that water has to work harder than solvents to accomplish the same cleaning task, it’s informative to compare the energy consumption of the two types of systems. But before we do, there is one important caveat: Cleaning systems come in myriad sizes, configurations and capabilities. Different soils and part geometries can dramatically affect the cleaning efficiency of a given system. As they say in the automotive ads, your mileage may vary.
In general, most solvent cleaning processes tend to be vertical, moving parts up and down in the cleaning system. Entirely self-contained, vapor degreasers usually are single-box machines about the size of a large desk or kitchen table. For example, a typical medium-sized vapor degreaser has outer dimensions of about 60 × 30 inches, with two cleaning tanks that are 10 inches wide, 12 inches long and 10–12 inches deep. Such a machine typically will use 3–5 kW of electricity per hour of operation.
In contrast, aqueous processes tend to be horizontal, moving the parts through a series of dip tanks. Aqueous cleaning systems have typically a 50–60% larger footprint than vapor degreasers of the same capacity, simply because of the need for more tanks, larger pumps, blowers, filters and so on. These machines typically use 8–10 kW of electricity per hour of operation.
Here is a very useful comparison from a supplier of both aqueous and solvent cleaning equipment. The company’s medium/large vapor degreaser costs around $50,000. It holds 50 gal of solvent and is 90 inches long × 36 inches deep. It uses 12 kW of power at startup, about 6 kW in continuous use and about a tenth of that power during stand-by mode depending upon the solvent used.
In contrast, the company’s aqueous system with comparable cleaning capacity costs around $85,000. It has four sumps and has a footprint almost twice as large—179 × 34 inches. It uses 17 kW of electricity during start up and 12 kW during use, operating at 140°F.
Many aqueous systems enhance cleaning with ultrasonics, adding a further 200–500 W of power consumption per tank. But whereas most vapor degreasers have only one tank fitted with ultrasonics, aqueous cleaning systems generally have three or more. So, ultrasonics add roughly another 1–2 kW of energy consumption to aqueous systems.
Auxiliary Equipment
Aqueous systems are not single-box designs. Auxiliary equipment required in addition to the basic three- to five-tank washing and rinsing system includes a deionized (DI) water system, some type of dryer and a wastewater treatment system. Each auxiliary process has its own energy requirement.
A typical aqueous batch system has one wash tank and two or three rinse tanks that require 2–5 gpm of DI water. The DI system also needs to heat the water to operating temperature; typical aqueous systems cannot tolerate large influxes of cold water. Assuming a cleaning temperature of 140°F, the deionizer will need at least 2–3 kW of power simply for purification and heating, and more for the pumps and support equipment.
At the other end of the system, parts need to be dried. Infrared heaters, blowers, turbo-blowers and air knives are used. On a typical aqueous cleaning system, any of these drying approaches can easily use 5 kWh. That number could double on a bigger machine simply because of the larger motors, fans and compressors required.
| Table 2: Comparative Energy Costs Of Solvent And Aqueous Cleaning Systems | ||
| Task | Vapor Degreaser (kWh) | Aqueous Degreaser (kWh) |
| Deionize and heat water | 0 | 1 |
| Operate degreaser | 4 | 8 |
| Drying processes | 0 | 5 |
| Wastewater treatment | 0 | 4 |
| Total electrical consumption/hr | 4 | 18 |
| Total operating consumption/month | 640 | 2,880 |
| Stand-by electrical consumption/day | 16 | 48 |
| Stand-by consumption/month | 512 | 1,536 |
| Total system electrical consumption | 1,152 kWh | 4,416 kWh |
| Total operational cost | $109/month | $420/month |
Wastewater treatment is a very complex issue because of the wide variety of processes and options available. In addition, some plants have suitable facilities already in place, so the extra energy consumption of waste treatment for a cleaning process may not be significant.
However, if the primary use of the water treatment system is to support the aqueous cleaner, then the energy costs and footprint of the waste treatment system are opportunities for savings. Assuming the system needs to process 5 gpm of wastewater, even the most frugal waste treatment system is going to use 3–5 kW of power. In general, aqueous cleaning systems always add cost and burden to plant treatment facilities.
Each of these auxiliary systems also adds heat to its surrounding environment, increasing the load on plant HVAC systems. The differences here are striking: the vapor degreaser mentioned above will add about 82,000 Btu/hr of heat to the room in which it is operating, while the aqueous system will add nearly 300,000 Btu/hr. The aqueous system also will add approximately 15 lb (roughly 2 gal) of water into the plant air of the plant every hour, which will need to be removed by the HVAC system.
One last consideration is stand-by power draw—that is, use of electricity to keep the machine ready for operations. In order to minimize solvent losses, vapor degreaser refrigeration units should be kept operational at all times. This generally requires 0.5–1 kW/hr of electricity. But at many companies, the heaters on aqueous cleaning systems are never shut down because of the long delay in reheating the water. This means the system uses 2–5 kW of electricity, hour after hour, day after day, even when not in use.
As shown in Table 2, the final tally shows vapor degreasers to be substantially more energy efficient than aqueous cleaners. Figures are based on 20, 8-hr work days each month and four, 48-hr weekends each month, with an electricity cost of 10.5¢/kWh.
Solvent Safety
Saving $300 a month may not seem like much compared with the overall operating budget of your company, but it’s a 75% reduction in cleaning costs and is a direct and instant reduction in the cost of goods sold. Which brings a bigger question into sharp relief: Given the inefficiencies of aqueous cleaning, how could it have become the predominant force in the market while vapor degreasing became relatively rare?
The answer is found in the global effort to protect stratospheric ozone. By the 1980s, the science of the ozone “hole” was fairly well-defined. With the ratification of the Montreal Protocol, most countries began a brisk phase-out of ozone-depleting solvents. Many of the most popular solvents used in vapor degreasers at the time were ozone-depleting substances, so when those solvents were pulled from the market those cleaning machines were rendered unusable. This gave aqueous cleaning its big opportunity.
Those zone-depleting solvents are a distant memory. Today’s solvents are ozone-safe, relatively non-toxic and nonflammable. While solvents are more expensive on a “per pound” basis than water, they are roughly comparable in cost when you account for all the surfactants and saponifiers used in aqueous cleaning chemistries.
The issue is not the solvent cost per pound but the cost per part cleaned. Unlike aqueous systems, vapor degreaser solvent consumption is measured in pounds per week instead of gallons per hour. In addition, vapor degreasers are designed to concentrate soil and contaminants, minimizing waste disposal. Aqueous systems generally dilute contaminants, making waste disposal more complex and costly.
Acknowledgements
The author would like to thank Wayne Mouser of Forward Technologies, Jon Harmon of Branson Ultrasonics, Art Gillman of Unique Equipment, Bill McCormick of Tiyoda-Serec Corp. and numerous other contributors whose expertise and judgments were incorporated into this article.
RELATED CONTENT
-
Smut and Desmutting
Question: I am new to this industry and have heard about smut and desmutting operations.
-
Blackening of Ferrous Metals
The reasons for installing an in-house cold blackening system are many and varied.
-
Choosing and Troubleshooting Copper Electroplating Processes
Learn more on this inexpensive and highly efficient process.