The Electroless Deposition of Nickel-Phosphorus-Tungsten Alloys
This paper describes the successful electroless co-deposition of tungsten within a nickel-phosphorus matrix to yield several advantageous properties.
#research
by Vijaykumar Ijeri, Snehal Bane, Komal Shah and Prerna Goradia, Grauer & Weil (India) Ltd.
(A printable PDF version of this paper is available by clicking HERE.)
Featured Content
ABSTRACT
Electroless nickel-phosphorus coatings are used on metallic components to enhance corrosion resistance, wear resistance and durability. Further improvement in properties can be obtained by adding additional alloying elements or nanoparticles. Tungsten is a hard, robust metal with high melting point and good resistance to acids and alkalis. However, it is a difficult metal to deposit. This paper describes the successful electroless co-deposition of tungsten within a nickel-phosphorus matrix to yield several advantageous properties. In particular, the Ni-P-W deposits obtained by the electroless process have resultant hardness in the range of 750-850 HV which increases on annealing. Tungsten content of 2-4 wt% is found in the deposits under optimized operating bath conditions. Further, the effect of operating parameters, like pH and temperature, on the rate of deposition, alloy composition and other surface properties are presented.
Keywords: electroless alloy deposition, nickel-phosphorus tungsten alloys, corrosion resistant coatings, wear resistant coatings
Introduction
The discovery of electroless plating is credited to Brenner & Riddell in the 1940s. Today electroless nickel (EN) plating has grown into a very substantial segment of the metal finishing industry. Electroless nickel coatings have been available to engineers and designers for more than five decades. This process has been called autocatalytic, chemical nickel plating or electroless nickel plating.
Electroless deposition can be done both on metals as well as nonconductors. Metals are easily coated with electroless deposits using hot baths. Nonconductors need to be activated with catalysts to initiate plating. This paper will focus only on the plating of mild steel.
There is a large variety of EN coatings, typically defined by their alloy content, the most common being Ni-P alloys. These deposits give a low coefficient of friction and are anti-galling. They have good as-plated hardness and can be further hardened by post-plating heat treatment processes. These deposits have excellent corrosion performance in many types of environments. The hardness and corrosion properties are very much dependent on phosphorus content. There have been studies on the relationships linking deposit phosphorus levels to the resulting hardness both before and after post-plating heat treatment processes.
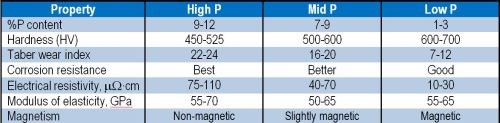
Wear performance has also been shown to be primarily a function of the deposit hardness, and this has been related to alloy phosphorus content. A few common properties are listed in Table 1.
Nickel-phosphorus deposition with a hypophosphite reducer is usually represented by the following reactions: (1,2) dissociation of salts, (3) reduction of nickel cations and (4) oxidation of hypophosphite:
1. NiSO4 + H2O → Ni+2 + SO4-2 + H2O
2. NaH2PO2 + H2O → Na+ + H2PO2- + H2O
3. Ni+2 + H2PO2- + H2O → Ni + H2PO3- + 2H+
4. H2PO2- + H2O → H2PO3- + H2
Table 1 - Comparison of properties obtained from different Ni-P alloys.
The main reasons for the widespread industrial use of electroless nickel plating are to be found in the unique properties of electroless nickel deposits. An advantage of electroless nickel plating is its ability to coat interior surfaces of pipes, valves and other parts on a variety of materials, including metals, plastics, glass and ceramics. Based on the excellent properties of the coatings, the electroless deposition of Ni-P has been widely investigated. Promising results on optimizing the characteristics of the Ni-P system by the introduction of a third element to form ternary Ni-P-based alloy coatings have been recently reported. The ternary systems that have been studied include Ni-Cu-P,1,2 Ni-W-P,3,4,5 Ni-Re-P and Ni-Zn-P.6
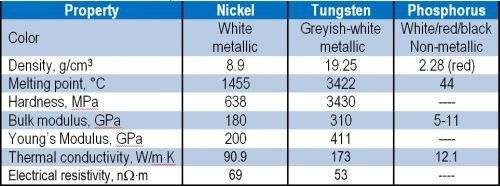
Among these, the introduction of tungsten appears attractive because of the unique properties listed in Table 2. Tungsten is a hard, robust metal with high melting point and good resistance to acids and alkalis. Co-deposition of tungsten within a nickel-phosphorus matrix is expected to yield advantageous properties.
Table 2 - Properties of individual elements for Ni-W-P.
Electroless Ni-P-W
Our Ni-P-W bath is essentially a mix of nickel and tungsten salts, with complexing agents, stabilizers and buffering agents. The bath is ideally operated at a temperature around 85°C under slightly alkaline conditions, pH ~8.2. With these conditions, the tungsten content in the deposits is ~3 wt%.
Tungsten cannot be deposited alone, either chemically or electrochemically. Co-deposition of tungsten with some of the other transition metals is possible. Electrochemically, it is possible to co-deposit tungsten up to about 70 wt% with nickel, but by an electroless process, it is much less, ~5 wt%. Nevertheless, even such a small amount of tungsten in the Ni-P film is sufficient to enhance the properties of the surface. It is known that phosphorus content has an effect on the hardness of the coating. A comparison of surface properties due to the presence/absence of tungsten in the deposit with similar phosphorus content can be appreciated from Table 3. These Ni-P-W deposits had a tungsten content of ~3 wt%. SEM images of the high-phosphorus (~12 wt%) deposits are shown in Fig. 1.
Table 3 - Comparison of Ni-P versus Ni-P-W coating properties.
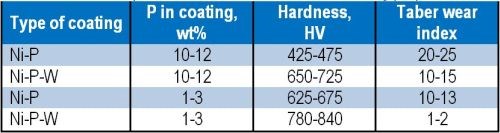

Figure 1 - SEM images of electroless deposits Ni-P-W (L) and Ni-P (R) with P= ~12 wt%.
In addition to the above properties, the corrosion resistance to neutral 5% salt spray was about 10 to 15% more for Ni-P-W coatings when compared to equivalent Ni-P deposits. When mild steel panels with ~10 µm thick coatings were immersed in 5% NaCl solutions, the regular low phosphorus coating developed red spots in 3 hr, while the regular high phosphorus (~12 wt% P) coating and the Ni-P-W (with ~6 wt% P) coating failed after 22 hr. From this, it is understood that incorporation of tungsten in the deposits increases the hardness as well as the corrosion resistance. The phosphorus and tungsten contents can be varied by changing the proportions of nickel and tungsten salts and complexing agents.
Effect of pH and temperature
A series of experiments was carried out to determine the effect of pH variation on the rate of deposition and the elemental composition. The pH was varied from 6 to 9, while the temperature was maintained constant between 85 and 87°C. Then a pH of 8 (optimum) was kept constant and the temperature was varied. The results are shown for constant temperature in Fig. 2, and constant pH in Fig. 3.
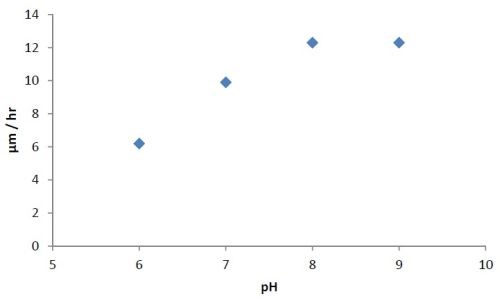
Figure 2 - Effect of pH on the rate of deposition at constant temperature (85-87°C).
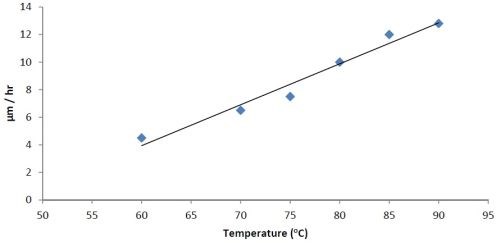
Figure 3 - Effect of temperature on rate of deposition at constant pH = 8.
The bath is unstable below pH 6 and it is difficult to maintain a pH above 9 at high temperatures. The phosphorus content in the coatings decreases from ~10.5 wt% to ~6 wt% as the pH is increased from 6 to 9. Concurrently there is a small increase in nickel content. The tungsten content doesn’t vary much, i.e., it remains around 2-4 wt%. The effect of varying temperature on phosphorus co-deposition is meager, but with aging of the bath, there is a steady increase of phosphorus content in the deposits. From these studies, it was concluded that the optimum temperature and pH should be 85°C and 8, respectively. With these conditions, one gets cauliflower-like deposits with ~3 wt% W. After heat treatment at 400°C for 1 hr or at 200°C for 4 hr in air, the hardness of the deposits increases from ~800 HV to ~1100 HV. Heating at 400°C causes a change in color, whereas heating at 200°C does not. There is also some oxide formation due to heat treatment in air. The surface morphologies of the deposit as-plated and after heat treatment are shown in Fig. 4. The EDS spectra for an as-plated coating is shown in Fig. 5.
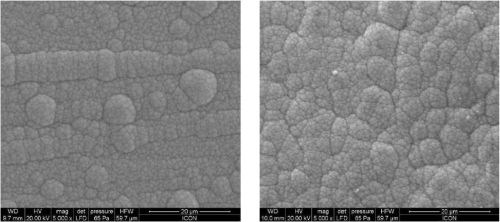
Figure 4 - Morphology of Ni-P-W electroless deposits: as plated (L) and after heat treatment (R).
Wear resistance and hardness
High hardness often translates to high wear resistance. Resistance to wear is often cited as one of the features and a primary reason to utilize electroless Ni-P deposits for many applications. Ni-P alloys possess a natural lubricity, and so all electroless Ni coatings have some level of resistance to many types of wear situations. Depending on phosphorus content, Ni-P deposits can be amorphous (>11 wt% P), crystalline (< 4.5 wt% P) or a mixture of both (5-10 wt% P). The electroless Ni-P-W presented in this study falls in the last category. Heat treatment of these deposits at different temperatures and durations of time will cause structural changes, specifically crystallization through the formation of nickel phosphide (Ni3P), to occur in these deposits which are responsible for hardness improvement.
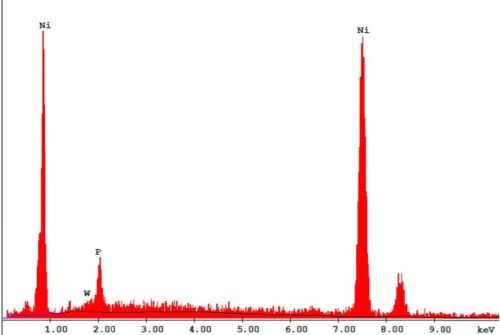
Figure 5 - SEM-EDS of a Ni-P-W electroless coating.
Wear can be considered as the progressive loss of material from a surface as a result of some relative motion between that surface and another one. In real-time wear interactions, considerations regarding metal-to-metal contact and the condition and hardness of the contacting surfaces must be taken into account. Wear testing can be complicated, both inside and outside of the laboratory. It has been accepted that if the ENP deposit is hard, or hardened by post-plating heat treatment, the deposit will have improved wear performance. However, this is not true in all cases. Wear is a function of both hardness and lubricity. Ni-P-W deposits showed a reduction in the coefficient of friction by almost half compared to regular electroless Ni-P deposits. Defining the wear resistance requirement for a deposit through the type of testing is important, but we must also acknowledge that testing any deposit in a comparative environment under similar conditions only serves as a predictor of performance under that specific set of conditions. Under identical conditions, the Taber Wear Index values of Ni-P-W deposits were nearly halved after heat treatment at 400°C for 1 hr.
The Ni-P-W coatings are strongly adherent to a variety of substrates following usual pretreatment practices and are also compatible with regular Ni-P deposits to build multilayer coatings (Fig. 6).
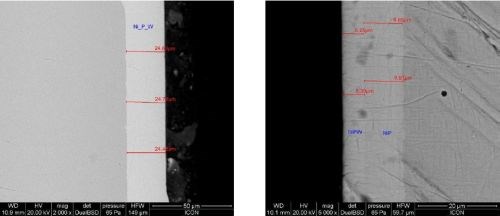
Figure 6 - Cross sectional SEM image of Ni-P-W electroless coating on steel (L) and double layer coating of Ni-P-W on high phosphorus Ni-P (R).
Typical applications are illustrated in Fig. 7.

Figure 7 - A textile winding drum (L) and piston rods (R) coated with electroless Ni-P-W.
Conclusion
Electroless Ni-P-W is an improvement over the traditional high/mid/low phosphorus deposits, as it confers improved hardness, wear resistance and corrosion resistance to the substrates from a single system which is not possible with regular ENP baths. More research into alloy depositions will certainly advance the chemistry and technology further.
References
1. Y. Wang, C. Xio and D. Zing, Plating & Surface Finishing, 83 (3), 57 (1996).
2. S. Armyanov, et al., J. Electrochem. Soc., 143 (11), 3692 (1996).
3. P. Nash, Phase Diagrams of Binary Nickel Alloys, ASM International, June, 1991; p. 235
4. B.W. Zhang, et al., Mater. Charact., 37 (2-3),119 (1996).
5. Y-Y. Tsai, et al., Surf. Coat. Technol., 146-147, 502 (2001).
6. M. Bouanani, et al., J. Appl. Electrochem., 29 (5), 637 (1999).
Note: Coating thicknesses were determined by x-ray fluorescence methods (CMI - Oxford Instruments) and by weight difference. Imaging of the surface morphology and alloy compositions were done by scanning electron microscopy (FEI - Quanta 200 ). Hardness measurements were done by nano-indentation (Fischerscope HM 2000). The coefficient of friction was measured with a Pin-on-Disk Tribometer (CSM Instruments). Finally, the wear index was measured on a Taber Rotary Platform Model 5135.
RELATED CONTENT
-
Gold and Silver Plating Basics
An overview of precious metal electroplating processes.
-
Blackening of Ferrous Metals
The reasons for installing an in-house cold blackening system are many and varied.
-
Aluminum Anodizing
Types of anodizing, processes, equipment selection and tank construction.





















