The Tribology of Contact Finishes for Electronic Connectors Part II: The Effects of Underplate, Topography and Lubrication
This paper is an expanded treatment of the second part of the William Blum Lecture Dr. Antler presented at Sur/Fin 1988 by Dr. Morton Antler. Subjects covered include substrate and lubrication effects on the tribology of contact finishes.
#research #surfin
By Dr. Morton Antler
Recipient of the 1987 William Blum AES Scientific Achievement Award
(Originally published as Plating & Surface Finishing, 75 (11), 28-32 (1988).)
Featured Content
ABSTRACT
The William Blum Lecture presented at SUR/FIN 1988 Los Angeles by Dr. Morton Antler, the 1987 AESF Scientific Achievement Award recipient, was expanded for publication in Plating & Surface Finishing by the author. This second concluding paper covered the effects of substrates and underplates on friction and wear. Part 1 of the 29th William Blum Lecture, entitled "The Tribology of Contact Finishes for Electronic Connectors: Mechanisms of Friction and Wear," was published in the February 2014 NASF Report in Products Finishing. This second paper also contains a summary of the salient points noted in both papers, providing continuity between the two articles published in the February and March 2014. A printable PDF version of this second part is available by clicking HERE.
Introduction
High hardness and ductility are desirable in contrast to the low-ductility top coating preferred for minimal adhesive wear and friction. Fluids of certain chemical classes, such as the polyphenylethers, are often essential supplementary films that assure low sliding friction and acceptable adhesive and fretting wear. New, low-cost contact finishes under development may be successful in the less critical applications of consumer electronic components. Coatings applied by physical methods such as ion implantation and ion plating are of interest for critical applications.
Substrate effects
Hardness - Hard materials are more wear-resistant than soft materials, provided that other characteristics of the metal are the same. This fact originates in the reduction in real area of contact which accompanies increasing hardness. Therefore, with layered materials, increasing substrate and underplate hardness may be beneficial, particularly when the coatings are thin.
Examples of the value of a hard underplate (e.g., nickel, 400 kg/mm2), are given in Fig. 1 for adhesive wear and in Fig. 2 for abrasive wear.1 In Fig. 1, hemispherically-ended solid gold riders with a diameter of 3.2 mm were mated to cobalt-gold plated flats at a series of loads for various numbers of passes on tracks 1 cm long. Adhesive wear of gold was much reduced with the hard underplate. In Fig. 2, a solid rhodium rider with a circular flat contact area 0.5 mm in diameter was mated to a lubricated cobalt-gold plated flat. Equiaxed graded silicon carbide particles were sifted onto the flat. The motion was reciprocating. The figure shows the abrasive-wear-inhibiting ability of the nickel underplate. Hard underplatings have also been found to be effective in reducing the fretting wear of gold electrodeposits.2
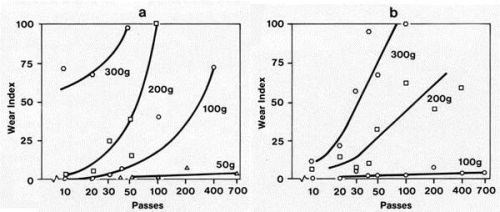
Figure 1 - Adhesive wear from runs with solid gold riders tested against a 3.3-µm-thick cobalt-gold electrodeposit on copper (a) without and (b) with a 2.5-µm-thick nickel underplate for the following loads: ⬡ = 300 g; ⬡ = 200 g; O = 100 g; Δ = 50 g.
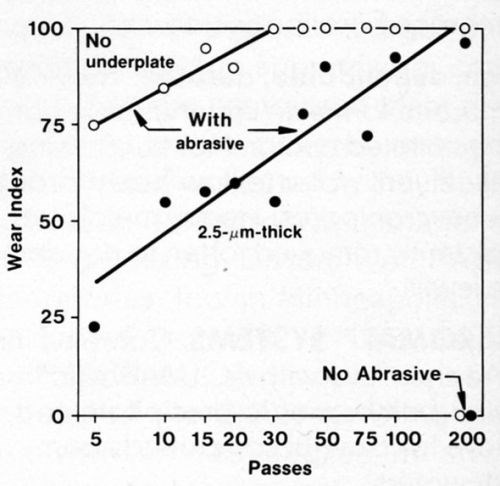
Figure 2 - Three-body abrasive wear runs with a 3.3-µm-thick cobalt-gold electrodeposit on copper with and without a nickel underplate (lubricated; 50-g load; coarse silicon carbide particles sprinkled on the flat).
Ductility - Underplates should be ductile so that they do not fracture during sliding, which would, in turn, cause porosity to develop in the gold or other metal finishes. For example, the brittleness of tin alloy containing 35% nickel underplate (elongation, 0.2%) has been the cause of failure of contacts plated with it despite its high hardness (700 kg/mm2). However, when the contact load is small enough so that the tin-nickel alloy remains intact during sliding, then a finish consisting of thin cobalt-gold on this underplate has very low wear and friction.3
Roughness - Smooth surfaces have been found to wear less than rough surfaces in both adhesive and abrasive wear.4 This is illustrated in Fig. 3, in which substrates were prepared at three levels of roughness (0.75, 0.38 and 0.1 µm c.l.a.) and were plated with the same mass of gold.
One reason why wear increases with increasing roughness is that it tends to occur preferentially on surface high spots. Thus, the gold is lost by wear first from the asperities. With smooth surfaces, wear is less localized than with rough surfaces, and more passes are required to achieve a given degree of wear.
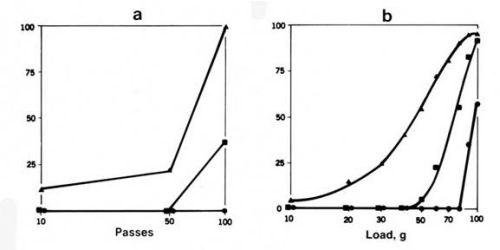
Figure 3 - Runs to determine the effect of surface roughness on wear for a 0.6-µm-thick cobalt-gold electrodeposit on a 1.5-µm-thick nickel underplate on copper flats abraded before plating to 3 levels of roughness (▲ 7.5 µm c.l.a.; ■ 0.38 µm c.l.a.; ● 0.1 µm c.l.a.). (a) adhesive wear with solid gold riders (100-g load); (b) two-body abrasive wear runs with a conical diamond rider (single pass).
Particular attention must be paid to roughness effects with nickel underplate. Nodular deposits can occur with poorly maintained baths, for example, as a result of inadequate filtration; to high current densities at edges and along scratches in the substrate surface; and with substrates that have particulate matter and precipitates on their surfaces. A nodular underplate will result in a nodular finish, and such surfaces can abrade an opposing contact that is softer. Since nodularity is also thickness-related, it is good practice to specify only as thick a nickel plating as is necessary to provide adequate support and the reduction in wear because of nickel's hardness for systems such as those illustrated in Figs. 1 and 2.
Adhesion - A plating will wear poorly if the stresses which result from sliding cause separation at the interface between the deposit and the underlying metal (whether it is an underplate or the substrate). The adhesion should be good even at the microlevel, and an example of a deposit having small non-adherent regions in the wear track is given in Fig. 4.
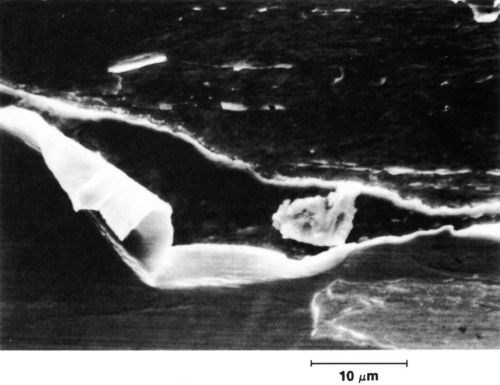
Figure 4 - Effect of sliding on a poorly adherent deposit. Plating is disrupted and porous. Two-body abrasion: 3.3 µm pure gold electrodeposit on copper. Diamond slider, one pass at 100 g.
Tribology of thin rim metallic contact lubricants
Thin films of some metals on thicker layers of other contact metals, or even of substrates which constitute the structure of the contact, have recently become commercially important. An example is the gold flashed palladium finish. When nickel is plated between the palladium and the substrate, the following terminology is used: gold flash is the plating; the palladium is the underplate; and nickel is the subplate. Strike deposits, which usually are used to promote adhesion between layers, are ignored in this discussion.
The primary function of the gold flash is to reduce friction and wear. It is an easily sheared coating, or metal film solid lubricant, for the palladium. The latter has intrinsically poor adhesive wear properties.6 This is readily understood by referring to Fig. 5. The hard palladium and nickel subplate support the load, with shear occurring in the gold layer. Pure gold, being soft, performs this function well, as expected. However, a cobalt-or nickel-gold has been found to be even better; they are relatively brittle and resist junction growth as described previously.7
A consequence of the physics of contact in Fig. 5 is that if the gold plating is soft and thicker than about 0.05 to 0.15 µrn, friction and wear will rise with increasing thickness. If the gold is thinner than about 0.05 µm, friction also will rise with decreasing thickness. For the thin gold coating to be effective, the substrate must be very smooth. Wear and friction parallel each other very well. A practical example is given in Fig. 6, in which the rider contact was a gold-palladium-silver inlay (DG R-156), and the flats were plated in a range of thickness with pure and with cobalt-gold.8 The palladium underplate was 1.5 µm thick on a subplate of 1.25 µm of nickel.
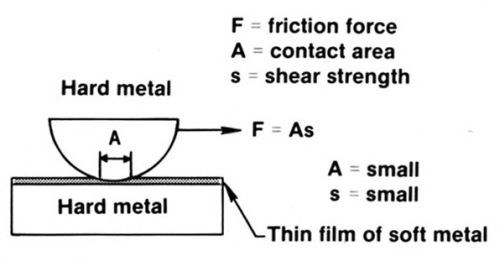
Figure 5 - The friction between metal surfaces can be lowered by depositing a thin film of a soft metal on a hard metal substrate (after Ref. 5). A coating of a metal that resists junction growth, such as electrodeposited cobalt-gold, will function in the same way as a soft metal film.
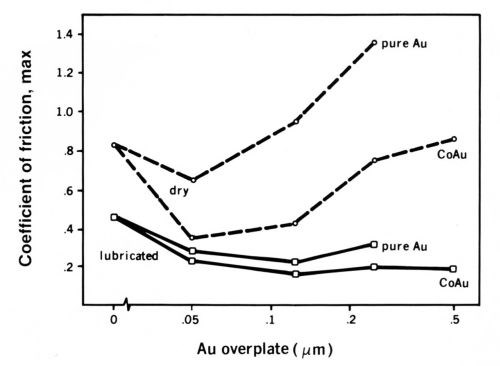
Figure 6 - Relationship between the maximum values coefficient of friction in runs with DG R-156 coated riders vs. coupons having gold on a palladium underplate with a nickel subplate. The variables were the type of gold plate (either pure or cobalt-hardened) and its thickness, and whether the flats were dry or were lubricated with a thin layer of a polyphenylether fluid.
Surfaces lubricated with a poly-phenylether fluid (discussed in the next section on organic lubricants) performed even better. Gold flash is commonly used on palladium nickel electrodeposits,9 and has been proposed for electroplated palladium silver,10 ruthenium,11 nickel phosphorus12 and other deposits.
Organic lubricants - Organic lubricants are widely used on electrical contacts. Their purposes are to: (a) lower friction; (b) control sliding wear; (c) inhibit corrosion in aggressive atmospheres; (d) reduce contact degradation resulting from the fretting corrosion of base metal finishes and substrates if the gold is worn through and (e) minimize contact resistance instability resulting from frictional polymers when the finish is a platinum group metal.
In the cases of (c) and (d), the lubricant blankets the surface, thereby retarding the formation of insulating oxides. In (e) the lubricant disperses the polymers, thereby preventing their accumulation on the contact. The subject of contact lubrication has been treated in detail by the author.13,14 Preferred lubricants are the 5- and 6-ring polyphenylether fluids with which contacts are coated by immersion and withdrawal, spraying, roller coating and other methods from dilute solutions in a volatile solvent such as inhibited 1,1,1-trichloroethane. Solutions of from 0.5 to 2 wt% of lubricant are commonly used for purposes (a), (b) and (c) above, and from 5 to 20 wt% for (d) and (e).
Lubricants are only marginally beneficial in reducing the friction and wear of tin and tin lead solder plated contact finishes. However, deposits having a hardness of at least 50 kg/mm2, such as most cobalt- and nickel-golds, respond well to them.
Needs and future developments
The marked dependence of the tribological behaviors of gold electrodeposits on the solution chemistry and bath operating conditions from which the platings are obtained is undoubtedly true of most contact finishes, including the increasingly important gold-flashed palladium and palladium-nickel alloys. Deposit performance is, of course, ultimately dependent on their structures, compositions and mechanical properties. The tools and techniques exist to explore these relationships, but thus far the metallurgists, physicists and mechanical engineers who make up the body of research tribologists have largely ignored the challenge (and complexity) of studying layered systems. Much of the work that has been done is largely empirical, involves optimization of the finish for particular contact-containing components, and is conducted in the secrecy that is necessary to maintain the legitimate proprietary interests of its industrial sponsors. Nevertheless, there has been some recent academic interest in the basic tribology of contact finishes. One example is a study15 of mechanical stresses using finite element methods, which has satisfactorily explained the author's observations' of the role of nickel underplate in reducing the adhesive wear of gold plate. Transmission electron microscopy has been proposed for studying the structural changes in golds that occur as a result of sliding.16 It seems appropriate for the American Electroplaters and Surface Finishers Society to identify new research projects on the tribology of contact finishes and to find university faculty interested in directing graduate level thesis research in this area. An AESF-sponsored fellowship through its Research Board on contact tribology would be timely.
Test apparatus - It is desirable to standardize on apparatus for evaluating the tribological properties of contact finishes, including their wear, friction and fretting behaviors. Component hardware can be coated with experimental finishes, but hardware generally does not readily permit the variation of important sliding conditions, such as normal force and wipe distance. In addition, the complexity of many contact shapes and the unique ways in which contacts can be mated makes it difficult to analyze hardware test results and to apply the findings to other systems. For many years, the author has used classical tribotesters which accommodate hemispherically ended riders, balls or rivets that are mated to flat coupons. Friction force and contact resistance are easy to measure with such apparatus. More recently, equipment which uses plated wires that are joined at right angles in a crossed rod configuration has been employed. American Society for Testing and Materials committees involved in contact work might consider writing guidelines for the design of wear test apparatus for materials, for their methods of use, and procedures for assessing wear.
Specifications on contact finishes - Since the tribological behaviors of finishes do not depend on simple characteristics such as composition and hardness, it follows that the plater and contact design engineer have, for the most part, been unable to write specifications having general utility. Platings are often called out in terms such as "Process X from Supplier Y has been found able to meet the requirements of the product," but (as in Fig. 7) can such a specification be divorced from the plating equipment that may be unique in a plant, and what else needs to be incorporated in these documents?17 Clearly, more work is needed to elevate our understanding of the properties of a contact finish on which tribological behavior depends.
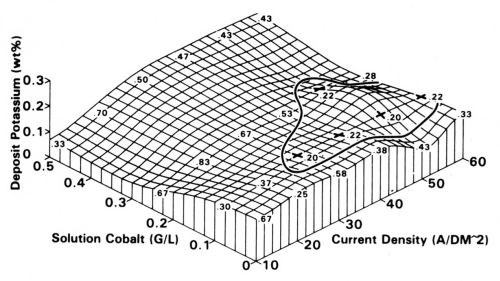
Figure 7 - Effect of cobalt concentration of electrolyte and cathode current density on potassium content of the deposit. (+) = experimental conditions resulting in "good" or "very good" wear. The numbers on the surface are the values of the coefficient of friction.
Cost reduction through new contact finishes
High reliability applications - Escalation in the price of gold began in 1972 when the need for electronic connectors was rapidly growing. Gold was the standard contact finish for high reliability, long-lived products. This spurred intense activity to reduce the dependence on this metal.18 The results of this research have been generally satisfactory. Selective plating, more realistic requirements concerning gold thickness and permissible as-plated porosity based on evaluations of field behaviors, better control of substrate roughness, and the acceptance of nickel underplate and of contact lubrication have all served to reduce the amount of gold that is necessary.
Recently, however, there has been a lessening in the willingness to consider non-gold contact materials; and even palladium and palladium nickel finishes are tribologically unsatisfactory without a gold flash, as discussed earlier. This reluctance is because of the need for (a) connector miniaturization, (b) an increase in the numbers of contacts in a connector, which usually involves reduced contact normal loads and a greater susceptibility of the contact to degrade by surface contamination19 and by fretting, and (c) increasing demands for reliable performance. The precious metal costs as a percentage of the manufacturing expense of a connector and of the total costs of the equipment in which they are used have been significantly reduced in the past few years. It is questionable whether new contact materials are likely to be readily accepted for the large volume-high reliability connector applications in telecommunications, computers and many other products. This is reflected in the lesser activity today in new contact materials research. If the cost of gold and palladium again rise dramatically and rapidly, and if such higher costs persist, then this situation may change. In the meantime, we have seen the focus of studies in the contact field return to basic phenomena and away from the search for new materials. Tribological research on contact finishes should be a part of this new emphasis.
Consumer electronics - Tin and tin-lead solder plate have always been important contact finishes for connectors in consumer products where lesser reliabilities and shorter product lifetimes are acceptable. The factors responsible for the persistence of these base metal finishes in consumer product applications are that such connectors (a) involve smaller numbers of contacts needed, (b) permit higher minimum contact loads (100 to 200 g) compared to that which is usual for connectors having precious metal contacts, (c) allow higher coefficients of friction and (d) are acceptable with smaller numbers of insertions than generally are required in other applications.
Consumer electronics are much more cost-driven than traditional electronics, and so there may be a greater willingness to explore lower cost finishes than gold and palladium where tin and solder are unacceptable, for example, because of a need for connectors having many contacts. New work on lubricated gold-flashed nickel and nickel-phosphorus electrodeposits12,20 suggests that it may be possible to achieve improved tribological behaviors and acceptable ancillary properties that are usual for tin and tin-lead solder plate.
An additional area of new contact materials research may involve thin precious metal finishes that have been treated so as to passivate their surfaces to protect them from degradation in aggressive atmospheres. It may be possible to obtain satisfactory lower cost finishes in this way and to achieve acceptable tribological behaviors as well by the supplementary use of lubricants. Early studies in this area might profitably be extended.21
Physical deposits - Deposits produced by physical methods include vapor deposition, sputtering, ion plating and ion implantation. All of them involve vacuum or plasma-assisted environments which can be attained only with large investments in capital equipment. Nevertheless, ion plating and ion implantation have attracted considerable attention. Ion plating is commercially successful for aerospace ball bearings.22 These deposits do not have a sharp interface to the substrate. Rather, they are graded, which gives them better adhesion to some substrates than can be achieved by electrodeposition. Gold is a material of choice. It acts as a thin film solid lubricant much as does the gold flash on electroplated palladium and other deposits. Ion plated gold should be considered for critical contact applications where superior tribological performance is needed, especially when supplementary organic contact lubricants cannot be used.
Ion implanted contact materials have been explored in a cursory way.23,24 The results have been promising, and this technique for obtaining hard, wear-resistant surfaces having low contact resistance again suggests that applications may be developed in the future in which their high process costs can be justified.
References
1. M. Antler & M.H. Drozdowicz, Bell System Tech. J., 5B (2), 323 (1979).
2. M. Antler & M.H. Drozdowicz, Wear, 74 (1), 27 (1981).
3. M. Antler in Corrosion Control by Coatings, H. Leidheiser Jr., ed., Science Press, Princeton, NJ, 1979; p. 115-133.
4. M. Antler, Insulation/Circuits, 26 (1), 15 (1980).
5. P.P. Bowden & D. Tabor, The Friction and Lubrication of Solids, Vol. 1, Clarendon Press, Oxford, 1950; p. 111-121.
6. M. Antler, ASLE Trans., 5, 248 (1968).
7. M. Antler, Plating & Surface Finishing, 75 (10), 46 (1988).
8. M. Antler & M. Feder, IEEE Trans, on Components, Hybrids, and Manufacturing Technology, CHMT-9 (4), 485 (1986).
9. J.P. Bare & A.M. Graham, Proc. IEEE Holm Conference on Electrical Contacts, p. 147 (1985).
10. F.I. Nobel, IEEE Trans, on Components, Hybrids, and Manufacturing Technology, CHMT-8 (1), 163 (1985).
11. R.G. Baker & T.A. Palumbo, Plating & Surface Finishing, 69 (1), 66 (1982).
12. C.A. Holden, et al., Proc. IEEE Holm Conference on Electrical Contacts (1988).
13. M. Antler, IEEE Trans, on Components, Hybrids, and Manufacturing Technology, CHMT-10 (1), 24 (1987).
14. M. Antler, ibid., p. 32 (1987).
15. A.G. Tangena & P.J.M. Wijnhoven, Wear, 121 (1), 27 (1988).
16. S.J.N. Goodman, private communication.
17. M. Antler, Products Finishing, 34, 56 (Oct. 1969).
18. M. Antler, IEEE Trans, on Parts, Hybrids, and Packaging, PHP-11, 216 (1975).
19. M. Antler in Treatise on Clean Surface Technology, Vol. 1, K.L. Mittel, ed., Plenum Press, New York, 1987; p. 179-203.
20. C.A. Holden, H.H. Law & G.R. Crane, Proc. IEEE Holm Conference on Electrical Contacts (1988).
21. M. Antler, Plating, 54 (8), 915 (1967).
22. M. Antler & T. Spalvins, Gold Bulletin, 21 (2), 59 (1988).
23. M. Antler, C. Preece & E.N. Kaufman, IEEE Trans, on Components, Hybrids, and Manufacturing Technology, CHMT-5 (1), 81 (1982).
24. P.W. Leech, ibid., CHMT-11 (1), 16 (1988).
Appendix: Highlights of Parts 1 & 2
This particular article was the second part of an extended paper contributed by Dr. Antler based on his William Blum Lecture. In order to provide added continuity between the two papers, Dr. Antler went further and prepared the following summary of the salient points discussed in the two articles.
1. Contact materials in electronic connectors, slip rings, switches and other components which serve low-voltage, low-current circuits in telecommunications, computer and similar applications are mainly electrodeposited finishes and claddings. Gold and its alloys are the most common, although palladium, tin, tin-lead alloys and a few other metals are used to some extent. The tribological properties of these materials control their functional behavior and reliability, and metal finishing practices, to a large extent, have been molded by tribological considerations.
2. Adhesive wear results from the formation of strong bonds at touching asperities, especially with clean surfaces. This can occur in mild or severe regimes according to load and can differ significantly in the extent of surface damage. The adhesive wear process, called prow formation, of many contact metals entails transfer from the member with the larger surface involved in sliding to that with the smaller surface, from which it subsequently can appear as loose debris. Prows consist of overlapping thin layers of metal.
When sliding occurs repeatedly in the same track, there is a transition to a process called rider wear in which, in the severe regime, only the smaller member loses metal by transfer. This transition depends on the material, load, track length and surface roughness, usually occurring within the expected lifetime of components.
3. Ductility is the key to determining whether high friction and wear by prow formation will occur. Golds, such as those containing cobalt from cyanide baths which have an elongation of about 0.4%, are less subject to prow formation and are, therefore, preferred to ductile finishes for sliding contacts. Codeposited polymers in these golds are believed to be responsible for their low ductility as well as their hardness.
4. Deposits can be so brittle, however, that they fracture during sliding. This is a cause of failure of the tin alloy electroplate containing 35% nickel, which has been used to some extent with a gold flash as a contact material.
5. Golds obtained at pulsed conditions appear to be more subject to adhesive wear than DC-plated golds which are obtained from the same baths.
6. Plating processes, especially from high speed systems, often have narrow "windows" from which deposits with satisfactory sliding properties can be obtained. A determination of these windows is necessary for plating process control.
7. Abrasive wear is caused by the plowing of a surface by an opposing member that is rough and substantially harder. Deposit nodularity is a type of roughness and should be avoided, especially with relatively hard metals such as nickel, which is commonly used as an underplate in contact finishes.
8. Fretting wear is the result of small amplitude oscillatory movement when mated contacts are subjected to vibration or other mechanical stresses. Wear debris accumulates between the contacts and, if base, will oxidize and cause the contact resistance to increase. In this case, the process is termed fretting corrosion.
9. Tin, tin-lead alloy, and other base metals are particularly subject to degrade by fretting. These finishes, when mated to gold contacts, have even less stable contact resistance behaviors during fretting than when the metals are mated to themselves.
10. Frictional polymerization occurs during the sliding and fretting of palladium and other platinum group contact metals. It results from the catalytically induced formation of tough organic films on the surface which originate in adsorbed air pollutants. These films are insulating and can be a cause of unreliability during fretting of connector contacts.
11. The gold flash commonly used on palladium and palladium-nickel alloy deposits is only marginally useful in protecting contacts from degradation resulting from frictional polymers when the finish is mated to itself. Fretting corrosion also occurs when noble contact finishes on base substrates wear through.
12. Palladium and gold-flashed palladium contacts mated to thick gold or gold alloys which are softer are stable during fretting. This is a result of metal transfer which converts the palladium contacts to all gold.
13. Hard ductile substrates and underplates are desirable, for they can lower adhesive, abrasive and brittle fracture wear and can minimize the rise of contact resistance during fretting in some cases when thin noble metal coatings are used.
14. Contact surfaces should be smooth when thin noble metal finishes are employed. In addition to minimizing as-plated porosity, such surfaces resist both adhesive and abrasive wear.
15. Non-adherent deposits wear poorly.
16. Thin films of relatively soft or of brittle metals, such as a pure- or a cobalt-gold flash on a harder substrate such as palladium, can reduce the friction and adhesive wear tendencies of the system. A gold flash has been proposed for use on ruthenium, nickel phosphorus and other experimental contact finishes.
17. Organic lubricants are widely used on electrical contacts. Their purposes are to (a) lower friction, (b) control sliding wear, (c) inhibit corrosion in aggressive atmospheres, (d) reduce contact degradation resulting from the fretting corrosion of base metal finishes and substrates if the gold is “worn through” and (e) minimize contact resistance instability attributable to frictional polymers when the finish is a platinum group metal.
18. Deposits obtained from physical processes, such as gold by ion plating or noble metals implanted with boron, nitrogen and other elements, are wear-resistant and may be useful in contact applications, especially when organic lubricants cannot be used.
RELATED CONTENT
-
Methods and Formulas to Determine Internal Deposit Stress in Applied Metallic Coatings
Internal stress exists in electroplated and chemically applied metallic coatings. This paper reviews the test procedures for measuring deposit stress and the formulas used to calculate stress values. Many formulas used require modification to obtain actual internal stress values. Errors in this regard are examined and common mistakes are explained.
-
Black Chromium Finishing: Beauty and the Beast
Over the past 10 years, there has been commercial development of black chromium deposits from a trivalent chromium electrolyte. This paper will review the deposit characteristics and operational consideration of these similar, but different chromium plating deposits.
-
Electroless Nickel Coatings: Appearance, Gloss and Surface Morphology
For decorative coatings, appearance is the essential purpose for application, but also for functional surface finishes it becomes increasingly relevant as an added value on top of specified technical requirements. Appearance is affected by spectrum and intensity of incident light, roughness and morphology of the coating surface, optical properties of the coating material, eventual superficial oxide films, and individual perception. The predominant factor is surface roughness, which in turn depends on base material roughness, quality of substrate pretreatment, and nucleation and growth kinetics of the electroless nickel (EN) deposit. Interdependency of gloss measurements with roughness measurements and with chemical composition of coatings was investigated for new generation mid-P EN processes and compared to traditional ones.



















