Vacuum Deposition Processes
The deposition of a film or coating in a vacuum (or low-pressure plasma) environment. Generally, the term is applied to processes that deposit atoms or molecules one at a time, such as in physical vapor deposition (PVD) or low-pressure chemical vapor deposition (LPCVD) processes.
#vacuum-vapor
In a vacuum, gas pressure is less than the ambient atmospheric pressure. A plasma is a gaseous environment where there are enough ions and electrons for there to be appreciable electrical conductivity. Vacuum deposition is the deposition of a film or coating in a vacuum (or low-pressure plasma) environment. Generally, the term is applied to processes that deposit atoms or molecules one at a time, such as in physical vapor deposition (PVD) or low-pressure chemical vapor deposition (LPCVD) processes. It can also be applied to other deposition processes such as low-pressure plasma spraying (LPPS).
The vacuum in deposition processing increases the "mean free path" for collisions of atoms and high-energy ions and helps reduce gaseous contamination to an acceptable level. When establishing a plasma in a vacuum, the gas pressure plays an important role in the enthalpy, the density of charged and uncharged particles and the energy distribution of particles in the plasma. A plasma in a "good vacuum" provides a source of ions and electrons that may be accelerated to high energies in an electric field.
Featured Content
In PVD processing, these high-energy ions can be used to sputter a surface as a source of deposition material and/or bombard a growing film to modify the film properties. Ion bombardment effects can also be found in LPCVD. The plasma may also be used to "activate" reactive gases and vapors in reactive deposition processes and fragment the chemical vapor precursors in plasma-enhanced chemical vapor deposition (PECVD).
PVD
Physical vapor deposition processes are atomistic where material vaporized from a solid or liquid source is transported as a vapor through a vacuum or low-pressure gaseous or plasma environment. When it contacts the part, it condenses.
The vaporized material may be an element, alloy or compound. Some PVD processes can be used to deposit films of compound materials (reactive deposition) by the reaction of depositing material with the gas in the deposition environment (e.g., TiN) or with a co-depositing material such as TiC or even a combination of the two.
Typically, PVD processes are used to deposit films with thicknesses in the range of a few nanometers to thousands of nanometers; however, they can be used to form multilayer coatings, thick deposits and free-standing structures.
Vacuum evaporation
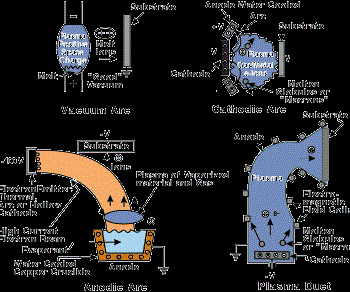
Vacuum evaporation (including sublimation) is a PVD process where material from a thermal vaporization source reaches the substrate without collision with gas molecules in the space between the source and substrate. The trajectory of the vaporized material is "line-of-sight." Typically, vacuum evaporation takes place in a gas pressure range of 10-5 to 10-9 Torr, depending on the level of contamination that can be tolerated in the deposited film. For an appreciable deposition rate to be attained, the material vaporized must reach a temperature where its vapor pressure is 10 mTorr or higher. Typical vaporization sources are resistively heated stranded wires, boats or crucibles (for vaporization temperatures below 1,500C) or high-energy electron beams that are focused and rastered over the surface of the source material (any temperature). Figure 1 shows several vacuum evaporation source configurations.
Advantages of vacuum evaporation:
- High-purity films can be deposited from high-purity source material.
- Source of material to be vaporized may be a solid in any form and purity.
- The line-of-sight trajectory and "limited-area sources" allow the use of masks to define areas of deposition on the substrate and shutters between the source and substrate to prevent deposition when not desired.
- Deposition rate monitoring and control are relatively easy.
-
It is the least expensive of the PVD processes.
Disadvantages of vacuum evaporation:
- Many compounds and alloy composi-tions can only be deposited with difficulty.
- Line-of-sight and limited-area sources result in poor surface coverage on complex surfaces unless there is proper fixturing and movement.
- Line-of-sight trajectories and limited-area sources result in poor film-thickness uniformity over large areas without proper fixturing and movement.
- Few processing variables are available for film property control.
- Source material use may be low.
- High radiant heat loads can exist in the deposition system.
- Large-volume vacuum chambers are generally required to keep an appreciable distance between the hot source and the substrate.
Vacuum evaporation is used to form optical interference coatings using high and low index of refraction materials, mirror coatings, decorative coatings, permeation barrier films on flexible packaging materials, electrically conducting films and corrosion protective coatings. When depositing metals, vacuum evaporation is sometimes called vacuum metallization.
Sputter deposition
Sputter deposition is the deposition of particles vaporized from a surface (sputter target) by the physical sputtering process. Physical sputtering is a non-thermal vaporization process where surface atoms are physically ejected by momentum transfer from an energetic bombarding particle that is usually a gaseous ion accelerated from a plasma or an "ion gun." This PVD process is often called sputtering.
Sputter deposition can be performed in a vacuum or low-pressure gas (<5 mTorr) where the sputtered particles do not suffer gas-phase collisions in the space between the target and the substrate. It can also be done in a higher gas pressure (5-15 mTorr) where energetic particles that are sputtered or reflected from the sputtering target are "thermalized" by gas-phase collisions before they reach the substrate.
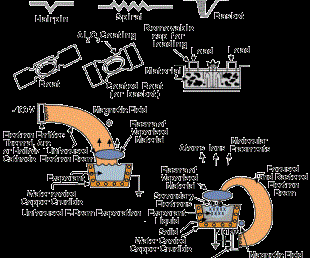
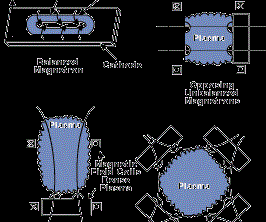
The most common sputtering sources are the planar magnetrons where the plasma is magnetically confined close to the target surface and ions are accelerated from the plasma to the target surface. In the unbalanced magnetron configuration, the magnetic field is configured to allow electrons to escape and form a plasma away from the target. The high sputtering rates attainable in magnetron sputtering allow reactive deposition of compound films as long as the sputtering target is not allowed to react with the reactive gas to form a low-sputtering rate compound (target poisoning). Figure 2 (see above) shows several sputter deposition configurations using planar magnetron sputtering sources.
Advantages of sputter deposition:
- Elements, alloys and compounds can be sputtered and deposited.
- The sputtering target provides a stable, long-lived vaporization source.
- In some configurations, the sputtering source can be a defined shape such as a line or the surface of a rod or cylinder.
- In some configurations, reactive deposition can be easily accomplished using reactive gaseous species that are activated in plasma.
- There is very little radiant heat in the deposition process.
- The source and substrate can be spaced close together.
-
The sputter deposition chamber can have a small volume.
Disadvantages of sputter deposition:
- Sputtering rates are low compared to those that can be attained in thermal evaporation.
- In many configurations, the deposition flux distribution is non-uniform, requiring moving fixturing to obtain films of uniform thickness.
- Sputtering targets are often expensive and material use may be poor.
- Most of the energy incident on the target becomes heat, which must be removed.
- In some cases, gaseous contaminants are "activated" in the plasma, making film contamination more of a problem than in vacuum evaporation.
- In reactive sputter deposition, the gas composition must be carefully controlled to prevent poisoning the sputtering target.
Sputter deposition is widely used to deposit thin film metallization on semi-conductor material, coatings on architectural glass, reflective coating on polymers, magnetic films for storage media, transparent electrically conductive films on glass and flexible webs, dry-film lubricants, wear resistant coating on tools and decorative coatings.
Arc Vapor Deposition
In arc vapor deposition, the vapor source is the vaporization of the anode or cathode of a low-voltage, high-current electric arc in a good vacuum or low-pressure gas. The usual configuration is the cathodic arc where the evaporization is from an arc that is moving over a solid cathodic surface.
In the anodic arc configuration, the arc is used to melt the source material that is contained in a crucible. The vaporized material is ionized as it passes through the arc plasma to form charged ions of the film material. In the arc vaporization process, molten globules (macros) can be formed and deposited on the substrate. To avoid this problem, a plasma duct may be used to bend the charged particles out of the line-of-sight of the source, and the macros will deposit on the walls of the duct. Figure 3 shows some arc vapor deposition configurations.
Advantages of arc vapor deposition:
- All electrically conductive materials can be vaporized.
- The arc plasma is effective in ionizing the vaporized material as well as reactive gases used in reactive deposition.
- Ions of the film material can be accelerated to a high energy before being deposited.
- There is little radiant heating (cathodic arc deposition).
- Reactive gases are activated in the plasma to aid in reactive deposition processes.
-
Poisoning the cathodic surface during the reactive arc vapor deposition is much less of a problem than with reactive sputter deposition.
Disadvantages of arc vapor deposition:
- Only electrically conductive materials can be vaporized.
- There is high radiant heating (anodic arc).
-
Molten globules (macros) ejected from the electrode can be deposited in the film, giving nodules on the surface.
Ion plating
Ion plating uses concurrent or periodic energetic particle bombardment of the depositing film to modify and control the composition and properties of the deposited film and to improve surface coverage and adhesion. The depositing material may be vaporized by evaporation, sputtering, arc erosion or other vaporization source. It can be obtained also from the decomposition of a chemical-vapor precursor species.
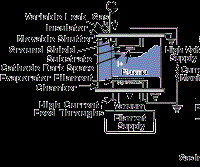
The energetic particles used for bombardment are usually ions of an inert or reactive gas or ions of the depositing material (film ions). Ion plating can be done in a plasma environment where ions for bombardment are extracted from the plasma, or it can be done in a vacuum environment where ions for bombardment are formed in a separate ion gun. The latter ion-plating configuration is often called ion beam assisted deposition (IBAD). Figure 4 shows two forms of ion plating, one in a plasma environment and one in a vacuum environment.
Advantages of ion plating:
- Significant energy can be introduced into the surface of the depositing film by the energetic particle bombardment.
- Atomic packing near the surface of the growing film can be densified by the concurrent ion bombardment (atomic peening).
- Surface coverage can be improved over vacuum evaporation and sputter deposition due to gas scattering and sputtering/redeposition effects.
- Controlled bombardment can be used to modify film properties such as adhesion, density, residual film stress, optical properties, etc.
- Film properties depend less on the angle of incidence of the flux of material deposited than they do on sputter deposition and vacuum evaporation due to atomic peening and sputtering/redeposition effects.
- Bombardment can be used to improve the chemical composition of the film material by bombardment enhanced chemical reactions and sputtering of unreacted species from the growing surface during reactive deposition.
-
In some applications, the plasma can be used to activate reactive species and create new chemical species that are more readily adsorbed to aid in the reactive deposition process.
Disadvantages of ion plating:
- There are many processing variables to control.
- It is often difficult to obtain uniform ion bombardment over the substrate surface leading to film property variations over the surface.
- Substrate heating can be excessive.
- Under some conditions, the bombarding gas may be incorporated into the growing film.
- Under some conditions, excessive residual compressive film stress may be generated by the atomic peening.
Ion plating is used to deposit hard coatings of compound materials, adherent metal coatings, optical coatings with high densities and conformal coatings on complex surfaces. Depositing aluminum films on aerospace components using ion plating is called ion vapor deposition.
Plasma-enhanced chemical vapor deposition (PECVD)
Chemical vapor deposition (CVD) deposits atoms or molecules by reducing the decomposition of a chemical-vapor precursor species that contains the material to be deposited. The reduction is normally accomplished using hydrogen at an elevated temperature. Decomposition is accomplished by thermal activation. The use of a plasma allows the reduction or decomposition to be done at a lower temperature than using temperature alone.
The deposited material may react with gaseous reactive species in the system to produce compounds (oxides, nitrides) or used in conjunction with PVD processes to produce compounds, such as carbide and carbonitrides, or alloys. Using plasma enhances the chemical activity of the reactive species, allowing chemical reactions to proceed at low temperature. CVD processing is generally accompanied by volatile reaction by-products, and those, along with unused precursor vapors and other processing gases, must be removed from the deposition system.
CVD processes have numerous other names, such as vapor phase epitaxy, when it is used to deposit single-crystal films; metalorganic CVD when a plasma is used to induce or enhance decomposition and reaction; low-pressure CVD when the pressure is less than ambient; and low-pressure PECVD when the pressure is low enough that ions can be accelerated to appreciable energies from the plasma. In some cases, the precursor vapor is not completely decomposed in the plasma, and the deposited film is in the form of polymeric changes. This process is plasma polymerization.
Some examples of precursor vapors and the materials to be deposited are:
SiH4 => Si, CH4 => C, NiCO4 => Ni, B2H6 or BCl3 => B, WF6 => W, TiCl4 => Ti.
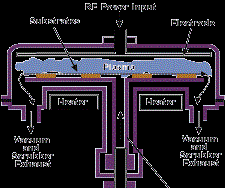
These can be combined with oxygen or nitrogen gases to form compounds and glasses. An example is the plasma-enhanced CVD deposition of phosphosilicate glass from silane, nitrous oxide and phosphine for encapsulation in the semi-conductor industry. Plasma-enhanced CVD can be used to deposit organic as well as inorganic materials. Examples are amorphous hydrogenated silicon for solar cells from silane, SiO2-x for permeation barriers from hexamethyldisiloxane and organic polymers form organic monomers. Figure 5 shows a RF-driven parallel plate plasma-enhanced CVD reactor such as used to deposit PSG glass.
Advantages of plasma-enhanced CVD:
- Many elemental, alloy, glassy and compound materials can be deposited.
- The microstructure of the material can be varied over a large range, sometimes from amorphous to polycrystalline to single crystal.
- High deposition rates.
- Complex surfaces can be coated uniformly.
-
Equipment is compatible with other vacuum processes.
Disadvantages of plasma-enhanced CVD:
- High deposition temperatures are usually required for complete decomposition or reaction.
- Some precursor material may be expensive, dangerous or unstable.
- Processing gases and vapors and by-products must be disposed of by the pumping system.
- There are many processing variables such as vapor concentration, gas composition, heating profile and gas flow pattern.
- Incomplete decomposition of the precursors can leave undesirable impurities in the deposited material.
In semi-conductor processing, this process is used to deposit insulator and encapsulating films, amorphous and polycrystalline silicon films and conductor metallizations. Low-pressure plasma-enhanced CVD is used to deposit diamond-like carbon films for wear resistance and is used in hybrid deposition processes to provide the reacting species.
Hybrid vacuum deposition processes
In some cases, two deposition techniques can be used at the same time or sequentially. One example is the use of sputter deposition of a metal in conjunction with low-pressure, plasma-enhanced CVD of carbon from acetylene to deposit a metal carbide as a wear-resistant coating on tools. If nitrogen is present, a carbonitride can be deposited. Varying the ratios of nitrogen and carbon in titanium carbonitride deposition can give a range of colors from black to purple to gold. These coatings are used for decorative and wear-resistant applications. Metal organic polymer composite materials can be deposited by a combination of evaporation or sputtering combined with plasma polymerization of an organic material.
Vacuum-deposition processing equipment
The equipment used to generate the deposition environment is an integral part of the process. The principle parts of the deposition system are the deposition chamber, fixturing, which holds the parts to be coated, and the vacuum pumping system, which removes gases and vapors from the deposition camber.
Generating a vacuum has two purposes: 1) To reduce the gas pressure enough so that vaporized atoms have a long "mean-free path" and do not nucleate in the vapor to form soot; and 2) To reduce the contamination level to the point that the desired film can be deposited. The fixturing holds the substrates to be coated and provides the motion, relative to the vaporization source. This is often necessary to give a uniform deposition over a large area, a complex surface or over many substrates. The fixture and process cycle times determine throughput. The deposition chamber is sized to contain the fixturing and provide room for accessories such as shutters, deposition rate monitors, heaters, etc. Proper design, construction, operation and maintenance are necessary to obtain a reproducible product with high yield and desired product throughput.
Vacuum deposition of thin films and coatings is continually evolving. This is true of processes, equipment, applications and markets. Often, the decision to use vacuum deposition processes is influenced by environmental concerns, since they are "dry processes." Developing applications include clear permeation barrier layers for polymer webs and three-dimensional containers, decorative/wear-resistant coatings for many applications, coatings to replace electroplated chromium, corrosion-resistant coatings to replace cadmium and others.
RELATED CONTENT
-
Ion-Vapor-Deposited Coatings for Improved Corrosion Protection
Applying highly-adherent aluminum coatings onto metals is recognized as a replacement for cadmium...
-
Extending Bath Life and Reducing Process Challenges in Vapor Degreasing Systems
How using a stabilizer can extend your bath life and help you avoid costly system repairs.
-
Mechanical Vapor Recompression Evaporation
MVRE is underutilized in the treatment of industrial wastewaters that are typical of metal fabricating and finishing industries. Increasing energy costs, rapidly decreasing freshwater resources, and growing sensitivity towards the environmental impact of industrial management practices are the driving forces in the development of more sustainable technology.