AES Research Project 3, Adhesion of Electrodeposits, Part 2, General Considerations
This is Part 2 of a four-part article consisting of the full report of AES Research Project #3, Adhesion of Electrodeposits, done at the University of Michigan in the mid-1940s, following the end of World War II. It outlines the general concepts involved on plate adhesion.
#basics
by
Featured Content
Project Director: A.L. Ferguson
Associate: Elmer F. Stephan
Chemistry Department, University of Michigan, Ann Arbor, Michigan
Part 2. General considerations
This is Part 2 of a four-part article consisting of the full report of AES Research Project #3, Adhesion of Electrodeposits, done at the University of Michigan in the mid-1940s, following the end of World War II. It outlines the general concepts involved on plate adhesion. A printable version of this section can be downloaded by clicking HERE.
Introduction
Adhesion of electrodeposited metals to the base metal has always been of major concern to the electroplating profession. Its importance has increased rather than decreased with time and even more so during recent years since thick electrodeposits have come into increasing general use through such processes as building up worn parts by iron, chromium and nickel and through the development of electrodeposited metals for bearings.
There are two major factors involved in adhesion; one is concerned with methods for securing adhesion and the other with methods for measurement. These are so intimately related that it is difficult to consider them separately, however, the present paper will be confined as closely as possible to the question of methods of measurement.
The whole matter of adhesion is further complicated by some factors of uncertainty that should receive consideration before entering upon the main topic of the paper. In the first place, one might ask what is adhesion? What is adhesion due to? What causes it? What forces are involved? In the second place, one might ask whether the conclusions arrived at concerning the methods for securing and for measuring adhesion of one electroplated metal to one base metal, or electrodeposited metals to different base metals. In other words, do different electrodeposited metals and different base metals possess personalities of their own and require individual attention. In the third place, one might ask whether the definition of adhesion should have universal application. In other words, may a deposit be said to be adherent for some purposes and not for others? Can one set of standards of specifications for relative degrees of satisfaction in adhesion be set up to apply to all cases, or should several sets be used each for a given purpose? And in the fourth place, one might even ask the question what is to be considered perfect adhesion. Strangely enough there is a distinct lack of agreement on the answer to this last most basic question. Each one of these factors will be briefly considered.*
The forces involved in adhesion
As pointed out above, one question which should be considered in any problem related to adhesion is, to what is adhesion due, or what are the forces involved in adhesion? From reading text books and published literature, one would get the impression that there are many different kinds of forces producing adhesion. Some of the more commonly named forces are: gravitational, magnetic, electrical, adhesive, cohesive, van der Waals', chemical, valence, covalence, atomic, etc. It is a very serious question as to whether these different names mean that there are many distinct kinds of forces or whether many of the different names are used simply for convenience or due to historical development, but actually refer only to different manifestations of the same force or different degrees of the same force. A whole book might be written on this subject, but attention will be drawn here to only a few points. It is just about universally accepted now that all matter is made up of molecules. Molecules of different kinds of matter are held together by so-called adhesive forces, those of the same kind by cohesive forces. Molecules are composed of atoms held together by so-called chemical, van der Waals’, electrovalent and/or covalent forces. Atoms in turn are made up of a central part which carried the mass of the atom and is positively charged, and an outer part made up of enough electrons or negative charges to neutralize the positive charge of the inner part. Since all matter is made up of a combination of atoms into molecules and, since pure material is only made up of the same kind of molecules, and since all other material since all other material is made only of different kinds of molecules, it is difficult to account for the existence of any basically different types of forces other than those found in the ultimate building blocks of all molecules, namely, the electrical and magnetic forces in the atom. If this point of view is accepted, then the different names for forces involved refer to different manifestations or degrees of intensity of the same basic forces within the atoms themselves.
The point of view expressed above that the forces involved in adhesion are identical with those involved in cohesion, adsorption, chemical reactions and in the, formation of crystals, in other words, that they are all atomic in nature is supported by much experimental evidence and many outstanding authorities in chemistry. One of the best known among these is Irving Langmuir, of the General Electric Company, Schenectady, NY. In one of his articles2 he makes the following remarks:
"In the past, it has been customary to consider that solids and liquids are held together by the "forces of cohesion" and are called "physical forces" as distinguished from chemical forces. In the following pages, the writer hopes to show that there is no present justification for this distinction between chemical and physical forces. Cohesion, adsorption and surface tension are all manifestations of forces similar in their nature to those acting between the atoms of solid bodies. It is therefore advantageous to look upon these forces as direct results of chemical affinity. The surface of a crystal or solid must be looked upon as a sort of checker-board containing a definite number of atoms of definite kinds, arranged in a plane lattice formation. The space between and immediately above these atoms is surrounded by a field of electromagnetic forces more intense than that between the atoms inside the crystal."
The following is one of the conclusions stated in this article by Langmuir:
"There is no present justification for dividing interatomic or intermolecular forces into physical and chemical forces. It is much more profitable to consider all such forces as strictly chemical in nature. Evaporation, condensation, solution, crystallization, adsorption, surface tension, etc. should all be regarded as typical chemical phenomena."
As part of another conclusion he states:
"A large number of experimental results are given which prove conclusively that adsorption is very frequently the result of the strongest kind of chemical union (primary valences) between the atoms of the adsorbed substance and the atoms of the solid."
Another independent type of evidence which also demonstrates that the forces of adsorption are chemical in nature is that gained from heats of adsorption. This evidence is summarized in a statement by S. Glasstone:100
"The chemical nature of the forces involved (in adsorption) is indicated by the heats of adsorption which are of the order of 20 to 100 kg-cal/mol, so that the bonds formed between the metal and the adsorbed gas are almost as strong as those existing in stable stoichiometric compounds."
It is a question why Glasstone uses the qualifying word "almost" since these heats of adsorption are within the range of heats of chemical reactions.
There are several instances known in which the strength of the force between the adsorbed material and the metal is greater than the cohesive force or tensile strength of the metal itself. A commonly presented illustration is the case of oxygen adsorbed on tungsten. In the course of time the normal oxide of tungsten WO3 distills from the tungsten surface and is deposited on the walls of the vessel. This proves that the so-called adsorptive force between the oxygen and the surface atoms of the tungsten wire is even greater than the so-called chemical force between these surface atoms of tungsten and the other tungsten atoms surrounding them.
It is universally accepted that the strength of a force increases with decrease in the distance over which it operates, the rate at which the strength increases with decrease in distance becomes much greater the shorter the distance, ultimately reaching a value of a high order of magnitude at interatomic distances. This means that the greatest manifestation of force strength is within the atom; the next in magnitude would be between the atoms within molecules, commonly called atomic or valence or chemical forces; the next would be between molecules or atoms of the same composition commonly called molecular or cohesive forces; and next those between molecules of different kinds, and commonly known as adhesive.
According to the modern concept of the structure of metals, only atoms are present. This means that all the forces, including those at the surface which result in adhesion are atomic or chemical in nature.
If, the above analysis is correct, then the degree of, adhesion for a given deposit and base metal becomes largely a matter of how intimate a contact can be made between atoms of deposited metal and the atoms of the base metal. It should he possible, theoretically, to make this contact so intimate that the force or bond between the deposited and base metals might be even greater, than the forces between the atoms of deposited metal or atoms of base metal themselves, which are commonly called cohesive forces. Or, to put it differently, the tensile strength of the bond might be greater than the tensile strength of either the deposited or base metal. This means that the greatest, secret to perfect bonding (the expression perfect adhesion is purposely avoided) is an absolutely clean base metal surface.
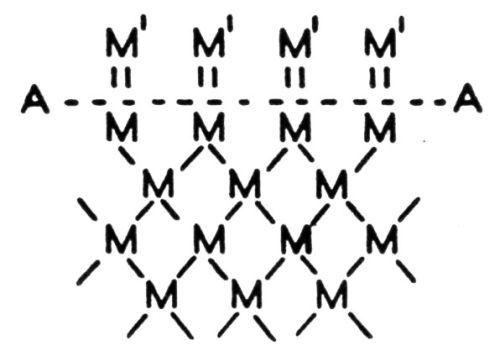
Figure 1 - Forces involved in adhesion.
One might illustrate the points involved in the above discussion by reference to the diagram in Fig. 1. The dotted line AA represents the surface of an absolutely clean base metal, the letter M represents atoms of the base metal, the lines connecting the M’s represent the forces between the atoms; M′ represents atoms of electrodeposited metal or some other, so-called adsorbed material which was adsorbed between the time of cleaning and the starting of electrodeposition, or some material that was not removed by the cleaning process. Within the body of the metal the atoms have all their forces satisfied, but on the surface, there must be forces which are not satisfied yet are of the same nature. These are represented by the vertical lines in Fig. 1 connecting the M and M′ atoms. These unsatisfied forces are extremely active and will tend to grab onto whatever comes along that will satisfy them. The author has frequently observed a freshly chemically cleaned surface acquire a visible tarnish within a few seconds. The presence, however, of a foreign material upon the surface of a base metal does not mean necessarily that a perfect bond is impossible with an electrodeposited metal. The surface forces of the base metal are fairly highly selective and even though they may be apparently satisfied by some impurity it is entirely possible that the atoms of an electrodeposited metal might more effectively satisfy them, in which case the previously bonded material would be displaced. This is a fact demonstrated by many experiments and extensively applied in some commercial processes including electroplating. It is probably a part of the explanation for the procedure of sometimes placing on the base metal a coating which preserves these forces until the plating process starts after which the coating is replaced by the electrodeposited coating and results in a perfect bond. An illustration of this is the anodic treatment of aluminum before the electrodeposition of another metal.
Perfect adhesion is not an absolute quantity expressible in psi
A second disturbing factor mentioned near the beginning of this paper has to do with the question as to whether conclusions arrived at concerning perfect adhesion resulting from a study of one electrodeposit upon one base metal may be generalized to apply to any deposit on any base metal. If the ideas presented above concerning the forces involved are accepted, then some generalizations may be permitted, but for many of the details each case of electrodeposition must be considered by itself, both from the point of view of nature of deposited metal and of the base metal. It may be stated, for instance, that a perfect bond may be obtained when the base metal is absolutely free from foreign material or when such foreign material may be replaced by the electrodeposited metal during the process of electrodeposition. However, the strength of this bond expressed in pounds per square in. cannot be taken as a standard for all perfect bonds. Every pair of metals would probably have a different numerical value, expressed in pounds per square in., for perfect adhesion. Neither can it be said that a method of producing a chemically clean surface on one metal will do so on another. How one base metal is handled between the cleaning tank and the plating tank may not apply for another. Composition of bath, temperature, agitation, concentration, current density, etc. are all factors which must be studied for each individual metal so far as adhesion as well as other properties of the deposit are concerned.
If the concept presented here concerning the forces involved in adhesion is true then it certainly is not possible to set up any absolute scale of perfect adhesion in pounds per square in. The maximum forces of attraction are distinctly specific for each pair of base and deposited metal. It might be possible to set up a standard in absolute units for each pair of metals provided a device were available by means of which the bond strength could be measured accurately. No such device has been perfected. As pointed out above and demonstrated in practice, the strength of a bond between the deposited and base metals may be greater than the strength between the atoms of the respective metals or the so-called tensile strength of the metals. In such a case, it would not be possible to measure the true bond strength. There are differences in opinions among authorities as to how to designate the adhesion when this is the situation.
Some experimental demonstrations of the existence of a bond strength that is greater than the tensile strength of one of the metals are contained in the article by Hothersall.94 Some extracts from this article follow:
"In previous work (A.W. Hothersall, Trans. Electrochem. Soc., 64, 83 (1933)), the author has shown that, when suitable cleaning treatments are used, a high tensile stress, acting at right angles to the general plane of the junction of deposit and base, is required to detach nickel deposits from a variety of basis metals. When fairly pure metals are used as a base, the stress required frequently approximates the tensile strength of the base, which is often less than that of the nickel deposit. Exceptions do occur and some of the causes for the basis metal, steel, will be discussed in a later part of this paper. Typical adhesion values using the Ollard method of test are given in Table 1."
Table 1 – Typical adhesion values for electrodeposited nickel on various metals.94
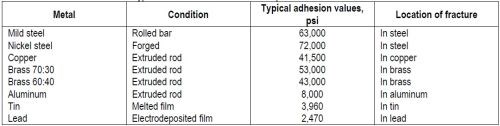
"Results of this high order, coupled with the fact that the fractures occurred not at the interface but entirely in the body of the underlying metal, prove that a strong bond existed between the two metals."
It will be noted that the fracture occurred in the base metal and not at the bond between plate and base metal. It should be pointed out, however, that this does not mean necessarily that the bond strength is greater than the normal tensile strength of the base metal. The surface layer of base metal may be weakened by various machining, rolling, polishing, etc. operations; or by pickling or other cleaning operations during which gases are liberated at or within the metal surface. The importance of these factors is well expressed in another paragraph from the same article by Hothersall.
"It must be remembered that adhesion tests of the type used in this work impose severe stresses upon the surface layer of the steel. Any weakness, even if only present to a depth of a few Angstrom units is as harmful as if the entire metal was weak. Machining or abrasion with sharp particles (emery) for example, probably leave the surface with minute tears and tracks and in a work-hardened and stressed condition. Polishing removes the torn surface but leaves a distorted layer below with a thin flowed film of amorphous structure at the surface, probably contaminated with polishing composition and oxide. Nickel deposits formed upon steel after such treatments are liable to be poorly adherent for two reasons: (1) the intrinsic weakness of the surface and (2) the additional weakness due to the embrittling effect of hydrogen discharged during cleaning and plating processes. As is well known from numerous researches of the effect of pickling and plating on the embrittlement of steel, this metal is especially susceptible to embrittlement when stressed. It is to be expected, therefore, that if the nickel is deposited on a stressed surface, the surface will become seriously weakened either by being embrittled or by the production of actual cracks; the effect of stress in the nickel deposit itself may aggravate the tendency of the embrittled surface to crack.”
The meaning of degrees of adhesion and perfect adhesion
The values in the above table and the accompanying remarks bring to attention the third basic disturbing factor involved in any discussion of adhesion mentioned at the beginning of this paper. It has to do with the very definitions themselves of such expressions as degree of adhesion and perfect adhesion. In discussions involving such expressions, some authors state definitely the meanings to be attached to them while others do not. This has led to much confusion. As an illustration, the following statements are quoted from a paper by Hothersall:94
"The term adhesion is used in this paper to indicate the force which must be applied to separate a deposit from the base on which it has been formed, irrespective of whether fracture occurs at the junction or elsewhere.
"The importance which is commonly attached to the degree of adhesion of an electrodeposited coating varies with the conditions under which the article is plated and subsequently used. In certain special applications of electrodeposition such as the building up of worn or under-sized parts, which has been successfully practiced in England for over twenty years, success depends largely upon the ability to produce, consistently, strongly adherent coatings which will withstand the severe stresses often applied in machining and in use. With decorative or protective coatings, which rarely exceed .002 in. in thickness, the plater is apt to consider the adhesion of his coating to be satisfactory if the normal percentage of rejects in the plating shop is kept down to reasonably small numbers. Apart, however, from the obvious advantages of working with an adequate margin of safety, it is important to consider the possible effects of poor adhesion on the serviceability of the article. To enable such effects of poor adhesion to be properly assessed, it is necessary to know something about the degree of adhesion which can be obtained and why the practice sometimes falls short of this ideal. This knowledge may also be important in enabling failures to be remedied or avoided and in assisting the development of improved methods to meet more stringent conditions of service.
"It is useful to remember, when attempting to correlate the results of adhesion tests such as are given in this paper with the behavior of coatings in practice, that the term 'adherent deposit' has no meaning other than that the deposit did not show any sign of separation under the conditions to which it was exposed. It is possible for thin, poorly adherent coatings to undergo what may appear to be a drastic test without signs of separation, the behavior in a given test often depending on a complex series of variables which include the thickness and mechanical properties of the deposit, the mechanical properties and surface roughness of the base. The tests used in this work were selected with the object of obtaining positive results as free as possible from such variables."
Attention should be drawn especially to Hothersall's interpretation of adhesion, that it is "the force which must be applied to separate a deposit from the base on which it has been formed, irrespective of whether fracture occurs at the junction or elsewhere." Also, the inference is clear that the expression "adherent deposit" might be applied to a given case when tested, by one method, or when a given use is in mind, while it might not be applicable if other tests were used or other applications anticipated.
In a communicated discussion of the paper by Hothersall from which the above quotations were taken, W. Blum makes the following comments on the interpretation to be placed upon "adhesion" or perfect adhesion:
"Tests involving deformation of the base metal may be influenced by the properties of the latter. However, to the extent that detachment of a coating may be expected to result from deformation in service, ability to withstand in a test the maximum possible deformation may be considered as evidence of satisfactory if not perfect adherence."
This statement indicates that Blum would qualify "perfect adherence" according to the particular use to which the deposit will be subjected in actual practice.
Later in his discussion Blum makes another highly significant statement concerning his interpretation of the expression "perfect adhesion."
"... reference is made to the effect of an intermediate film of a weak metal. A striking example was found in plating nickel over a thin layer of zinc or cadmium on steel. The nickel coating could be easily pulled, leaving a film of zinc or cadmium on both the steel and the nickel. In such a case, there is perfect adhesion, though the apparent adhesion is poor. In fact, it might be considered that, whenever fracture occurs in a base metal or undercoat, the adhesion of the outer coating is perfect by definition."
The highest type of bond between deposited and base metals
There is one more point of a general nature involved in any discussion of adhesion of electrodeposits to base metal. It was pointed out early in the paper that the forces involved are really chemical or possibly even better called atomic. It was also pointed out that the strength manifested in any given case depends upon how close the atoms come to each other and that the strength decreases at an increasingly higher rate with increase in distance apart, becoming practically zero when the distance is equal to a few molecular diameters. Probably the most familiar name given to the force involved in bringing about the bond between deposited and base metals is crystallizing force. The strongest possible bond should be, therefore, one in which the atoms of electrodeposited metal continue the same crystals as present in the surface of the base metal. Whether the continuation of such crystal growth ever takes place has been and still is a controversial subject. Another type of bond which should be of the atomic nature is the formation of a metallic compound or alloy between the deposited and base metals.
R.J. Piersol makes this statement:47
"In 1924, in an attempt to plate lustrous chromium directly on the polished surface of a spun flood-light reflector made from cold rolled copper, visual inspection showed that the grain structure of the copper was faithfully reproduced by the chromium and microscopic examination indicated that the crystal structure was carried through from copper to chromium."
The first study recorded in the literature of an attempt to demonstrate the influence of the structure of the base metal on the structure of the deposit is presented by W. Blum and H.S. Rawdon.10 They state that:
"It is shown that if copper is deposited electrolytically upon cast or rolled copper which has been cleaned in alkali, the structure of the base metal does not apparently affect that of the electrodeposit. If, however, the surface of the base metal has also been treated with nitric acid, the electrodeposited copper possesses both the crystal form and orientation of the base metal."
Fig. 2 contains photographs used to prove this statement.
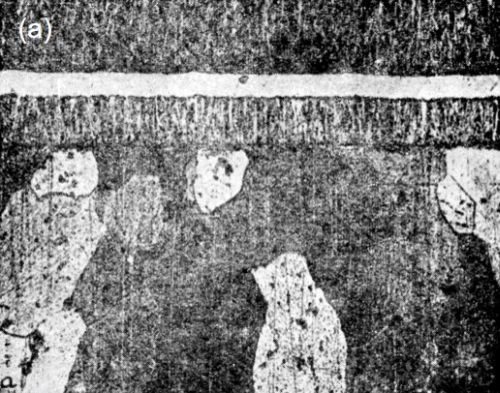

Figure 2 - Section of the chilled side of a “pig” of cast copper, upon which copper was electrolytically deposited (100×): (a) the surface was cleaned, but not pickled, prior to the electrodeposition, (b) the surface was pickled after cleaning. The various zones indicated in “b” are the same throughout all of the micrographs: w, base metal; x, electrodeposited copper (first layer; y, electrodeposited nickel; z, electrodeposited copper (second layer). [Editor’s note: w, x, y and z not clearly labeled in this figure as originally published.]
In an article11 by A.K. Graham, more evidence of the continuation of the crystal structure of the base metal in the electrodeposit is presented. Graham takes exception to the conclusion of Blum that the continuation of crystal structure depended upon a dip into a nitric acid bath. Graham concludes that:
"Reproduction, therefore, does not depend so much on the preliminary chemical treatment as on the state of the metallic surface."
Several microphotographs are presented which illustrate continued growth of the crystal structure of base metal into the deposit. Some of these are reproduced in Fig. 3.


Figure 3 - Copper deposit on annealed copper sheet, current 1 A/dm2: (a) showing reproduction of large grains; (b) showing large grained structure of the deposit. Both deeply etched.
The following remarks are taken from the discussion of the above paper by Graham:
Wm. Blum: "Such a distinction as Mr. Graham has made may be useful, even though it is not sharply defined. The general principles of crystal growth will probably determine the relation of the structure of any layers to those previously deposited, regardless of whether or not the initial layers owe their structure to that of the underlying base metal.
C.P. Madsen: "I do not agree with Mr. Graham's conclusions because two considerations have not been taken into account. First, he has omitted making deposits on ferrous metals; second, he has omitted nickel.
"In his first conclusion he says, 'Reproduction, therefore, does not depend so much on the preliminary chemical treatment as on the state of the metallic surface.' I think neither is correct. My opinion is that when the work is completed it will sum up something like this: that any electrodeposit tends to copy the structure of any metal. Whether it does so or not depends upon the condition of the metal surface and whether it has been cleaned by chemical or mechanical means.
"Furthermore, Mr. Graham is not justified in drawing a conclusion that the reproduction or tendency to reproduce the base metal structure is always undesirable. It may be desirable, for instance, to deposit a copper surface which copies the fine structure of a rolled copper base. On the other hand, it may safely be said that it is always undesirable for a nickel deposit to copy the structure of any metal other than nickel upon which it is deposited, because the natural structure of a pure nickel deposit is so fine that its hardness and tensile strength may be even greater than that of the best tool steel obtainable. Therefore, if the strength of electrodeposited metal is a function of the structure, as it is in metals made by melting, it is undesirable that nickel copy the structure of metals which are not as strong and hard as the best nickel structure obtainable electrolytically.
A. Kenneth Graham (communicated): "Replying to Mr. Madsen's remarks, deposits of nickel were studied; iron was not. I fail to see what bearing this has on the results stated in the paper.
"The statement that 'Reproduction does not depend so much on the preliminary chemical treatment as on the state of the metallic surface' is not an attempt to explain the mechanism of the observed phenomena. It is merely a contradiction of a statement made in a previous article to the effect that reproduction was obtained if the cathode (copper) were taken from a nitric acid dip into the plating bath, but not if taken directly from an alkaline (cleaning) solution.
"Regarding the last statement, I am unaware of making any statement to the effect that reproduction is undesirable. On the contrary, I would be tempted to believe that reproduction would cause greater adhesion of the deposit, with resultant advantages in cases of wear and friction, etc."
Instances of continuation of the crystal structure of the base metal into the deposited metal are very limited and many contend that such microphotographs have been misinterpreted. Additional evidence which appears conclusive is submitted by Hothersall.94 He refers to the articles by Blum and Graham and an earlier article by himself in which some skepticism is expressed. He claims now, however, to have shown that this phenomenon is not limited to metals of the same crystal system, but can also occur with metals of different crystal systems. He states:
"It has been suggested by workers in other fields that the evidence of such photomicrographs as [Fig. 4] falsely suggest a continuation of structure, but an x-ray examination made at Woolwich of several selected large crystals in a piece of cast copper showed an identical single crystal pattern both before and after deposition of a substantial thickness (about 0.01 in.) of copper.

Figure 4 - Continuation of microstructure of annealed silver by electrodeposited copper: upper, copper deposit; lower, annealed silver.
"The fact that continuation of microstructure has only been demonstrable with a limited number of electrodeposits is easily explained. For success with this method, it is essential that there should be little hindrance to the continued growth of a single crystal of the electrodeposit. A pure acid copper sulfate bath is suitable since this condition is fulfilled at low current densities. With nickel deposits, however, it has been found that, even under the most favorable conditions of deposition, non-metallic matter is included with the deposit to a sufficient extent to interfere with the continued growth of a given crystal. The growth of large grains is thus prevented and continuation of the structure of the basis metal cannot usually be seen under the microscope.
"It can, however, be shown indirectly that such continuation does occur over small thicknesses. Thus, a sample of annealed electrolytic iron was cleaned by degreasing and etching anodically in sulfuric acid and a thin layer of nickel (approx. 10-5 in. in thickness) was deposited under conditions which yielded maximum grain size (Watts' type single salt bath, 35°C, 5 A/ft2, pH 5.4), followed by a thick coating of copper from a pure acid copper sulfate solution at 35°C. with low current density (5 A/ft2). The microstructure of the iron (body centered cubic system) was copied by the copper deposit [Fig. 5] as already indicated, this implies that the orientation of the grains in the copper and iron are similar and therefore presumably in the intermediate nickel deposit also. When a thick nickel deposit was formed on a similar piece of electrolytic iron, it was found to have a columnar microstructure and it is suggested that, as the deposit grew, the orientation, initially copied from the iron base, gradually changed owing to the interference of foreign matter included in the deposit until, within a relatively small distance from the base, the deposit assumed the normal habit of electrodeposited nickel (i.e., with the majority of cube faces approximately parallel to the plane of the deposit). The greater the interference, i.e., the smaller the grain size, the more quickly is the orientation influence of the base likely to disappear. The results briefly reviewed above and the conclusions drawn from them indicate, therefore, that when nickel is deposited on a clean, etched surface of pure iron, it follows for a time the orientation of the crystal lattice of the iron and that the two metals are held together by atomic forces. With metals of commercial purity the evidence of adhesive forces, approaching or exceeding the tensile strength of the metal, suggests that this type of bonding applies generally to electrodeposits of nickel on clean surfaces."
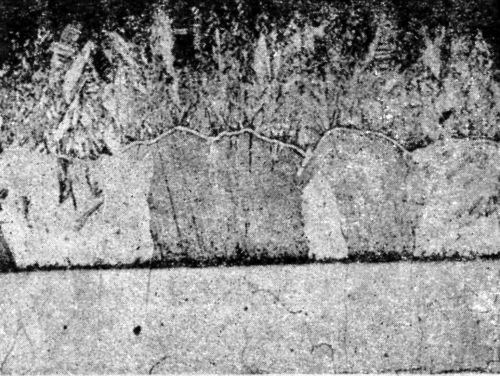
Figure 5 - Continuation of microstructure of annealed iron by electrodeposited nickel and copper: upper, copper backing; center, copper deposit; not visible, thin nickel deposit; lower, electrolytic iron annealed.
The most recent developments in this field have been made by C.L. Faust in connection with his work on electropolishing. In a recent paper (unpublished) he states:
"Thus, after electropolishing, the basis metal presents its true crystal structure to the depositing metal in the subsequent plating process. The plated metal can then continue the crystals of the basis metal as well as the crystal habits of the two metals will permit. A metal-to-metal bond is realized in which the atomic forces provide the adhesion. Better bond strength than that which results, cannot be obtained. The interatomic forces determine the strength of any metal, and it is the full operation of these that define the best bond strength that can he obtained in plated metals."
Polished versus etched surfaces for better bonding
Another highly controversial topic relative to bond strength has to do with the smoothness of the surface of the base metal. Many argue with much feeling that a more or less roughened or etched surface gives the best bond, and considerable evidence is available in support of this view. Many others argue with equal energy that the smoother the surface the better the bond. The former point of view is well-expressed by R.J. Piersol.47 His statements follow:
"In plating nickel on metallic aluminum, it is quite essential to etch the surface. Aluminum is subject to almost instant oxidation in the form of a very thin transparent film of aluminum oxide. Since the oxide surface is more resistant to etching than non-oxidized aluminum, which is exposed when once the surface is punctured, microscopic examination reveals the etched surface to appear as shown in [Fig. 6a]. Due to throwing power of a bath, such as nickel, the cavities are plated, resulting in a strong interlocking action so that the mechanical holding force of the plate to the foundation is of strength equal to the cohesive (tensile) strength of the deposited nickel.
"Instead of deep etching, an extremely fine surface etching may occur. The microscopic appearance of the boundary is shown in [Fig. 6b]. Since the strength of adhesion is dependent on the force per unit area, perpendicular to the plane of contact, necessary to pull the surface apart, it is evident that the effective strength of adhesion will be increased in proportion to the surface area of contact. In terms of atomic surface area of contact, surface etching may increase the effective contact area from two to four times."
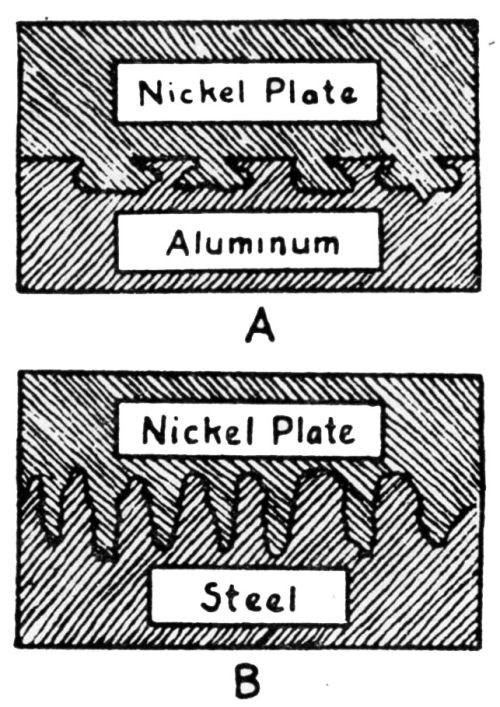
Figure 6 - (a) Illustrating the interlocking action of nickel plate on aluminum; (b) boundary appearance of nickel plate applied to finely etched steel surface.
The opinions of H.L. Crosby and L.I. Gilbertson are summarized in the abstract of their article.116
"Photomicrographic examination of silver electrodeposited on steel indicates that the adherence of silver to steel is dependent on factors other than mechanical interlocking. Evidence indicates that the strike produces crystal nuclei at the edges of ferrite crystals and that the columnar, usually twinned, crystals which are plated on silver-struck steel grow from these nuclei. It appears that the most important factor in the bond between silver and steel is the intra-atomic bond between closely associated crystals of ferrite and silver and that this condition is favored by the use of a silver strike before plating. Previous reports that a strike is essential to the best adherence are confirmed."
In an early article (1933) by Hothersall,43 the statement is made:
"The suggestion that mechanical interlocking is capable of giving rise to any appreciable adhesion is not supported by experimental results which on the contrary tend to negative the possibility of such a mechanism."
In a discussion of this paper,43 Blum has this to say in part:
"On page 6, paragraph 5, the author, Hothersall, discredits the beneficial effect of 'mechanical interlocking,' although all the researches on the plating of aluminum have shown the need for such interlocking to obtain even moderate adhesion. Would it not be more correct to say that if there is real atomic adhesion (i.e., cohesion), roughening cannot increase the bond; but when passive films are present, as on aluminum, no appreciable adhesion is possible except by interlocking."
In a later paper94 (1939), Hothersall provides the following statements and data:
"Suggestions have frequently been made that a mechanical interlocking of the deposit and base is responsible partly at least, for the adhesion of an electrodeposited metal. There is no doubt that mechanical interlocking can occur and that it can cause deposits to adhere when they might otherwise peel or flake, especially when they are of the order of thickness normally applied in decorative plating. It is, however, considered that mechanical interlocking can, at best, only contribute a small amount to the strength of the bond which can exist between many electrodeposits and the metals to which they have been applied. The author has obtained high adhesion results on polished surfaces which were only given a very light etching treatment, insufficient to dull the polish perceptibly. Examples are shown in Table 2.
Table 2 – Adhesion of electrodeposited nickel to polished (buffed) metals.
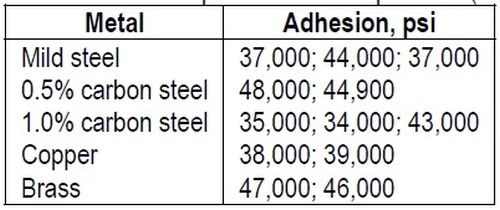
"These results cannot be explained by mechanical interlocking. On the contrary, an explanation involving some form of atomic bonding is necessary."
In connection with his recent work on electropolishing, Faust has shown that a steel surface cleaned in this manner, which is the smoothest kind of a surface, or by mild mechanical means, such as used by a metallographer, gives a bond with electrodeposited nickel having a tensile strength of 90,000 psi, as measured by the Ollard method which is the same as that of nickel or nearly that of the steel. Under corresponding conditions except without the electropolish, the bond strength was only 35,000 psi. Microscopic examination showed that the failure in the latter case was due to a weak surface layer in the steel. The electropolish removed this weak layer, and thus about doubled the measured bond strength.
F.C. Mesle96 makes the following remarks:
"It is generally assumed that the smoother the surface the more difficult it is to get an adherence of the deposit. Frequently, an acid dip is suggested to etch the surface to give the plate a chance for anchorage. This seems very logical if plating is held by a mechanical bond, and no doubt some plating is. But when this is so, the plating at best is poorly adherent. It is my experience that a perfectly adherent deposit can be plated on the most highly finished surface. In fact, under the same cleaning conditions, the adherence is better on the highly finished surface than on an emeried, brushed or satin finished surface.
"In making comparisons of the degree of adherence of any metal plated with any metal, I have found that the adherence is seldom better on the rough surfaces and many times the adherence was better on the smooth or highly buffed surface."
Extensive experimental evidence is contained in this paper by Mesle to demonstrate that the smoothest surface, irrespective of the base metal or deposited metal provides the strongest bond.
E.A. Ollard14 offers the following observation:
"In this connection, the action of the pickling bath is interesting, as it is difficult to see why so much better results are obtained by this method than by others. The first action of the bath is probably to remove all surplus matter and expose the crystal structure, after which the work goes passive and gasses.
"It is worth noticing that it has been found that if an electrodeposit is made on a carefully pickled metal its structure may follow that of the base (A.K. Huntington, Trans. Faraday Soc., 1, 324 (1915); Blum and Rawdon, Trans. Electrochem. Soc., 44, 305 (1923)). This seems to show that the crystals of the base metal exert some effect on those of the deposit.
"It is now questionable whether the adhesion can be due to the keying action of the nickel into the pickled base, or whether it is caused by the cohesion between the crystals of the two metals. From a microscopic examination, it does not seem probable that this action would be sufficient to account for the observed effects. It must, therefore, be ascribed to the cohesion between the crystals of the two metals, though whether this is due to molecular attraction, inter-mingling of the space lattices, or an inter-crystalline cement, can only be decided by the physicist. It seems probable, however, that if a crystal of deposit can be grown direct on to one of the base metals, the two will adhere in the same way and with forces of the same order as the crystals of solid metal."
In support of the etching treatment in order to secure better adhesion, R. Yates98 makes the following statement:
"In every instance where aluminum was plated without preliminary treatment of etching, the results were very sad indeed, and no record has ever been made of commercially acceptable adhesion being produced in any instance. Until about two years ago it was generally conceded, that there was no acceptable method of plating aluminum in the sense that other metals are plated."
Many more statements might be quoted concerning this topic, but enough have already been given to show the conflicting experimental evidence and the attitudes taken by authorities on the subject.
There are numerous other factors of a general nature which might well be considered in any treatment of adhesion, but no more will be discussed here. Some will be taken up in connection with the major topic of this paper which has to do with methods for measuring the degree of adhesion of electrodeposited metals on a base metal.
Footnote:
* In the discussion of these factors and in fact throughout the whole paper, extensive use will be made of the direct words of individuals as recorded in the literature. The objective is to make the literature search so exhaustive that any individual interested in the subject in the future might feel it is not necessary for him to make a similar survey. It is recognized that this objective is not attained 100%, and any additions to the bibliography will be much appreciated.
RELATED CONTENT
-
The Powder Coating Process
Powder coating is one of the most durable finishes that can be applied to industrial manufactured products, and offers excellent corrosion protection and is very safe because of its lack of volatile organic compounds. To understand the powder coating process you should start with the fundamentals.
-
Fixing Corrosion Between Anodized Aluminum and Steel
Anne Deacon Juhl, Ph.D., with AluConsult, says Galvanic corrosion is due to an electrical contact with a more noble metal or a nonmetallic conductor in a conductive environment.
-
Polishing vs. Buffing: What's the Difference?
Is polishing the same as buffing? Mechanical finishing expert, Pat Wenino, explains the differences between the two processes.


















