AES Research Project #41: Plating on Aluminum, Part 1: A Literature Review
Originally published in 1978, this paper was the first of several on AES Research Project #41, a study of plating on aluminum, at the National Institute for Science and Technology. A review of immersion pretreatments for the plating on aluminum is presented. The zincate pretreatments dominate the literature, but innovations were reported which offer improved adhesion and corrosion performance. Finally, an updated bio on Dr. Lashmore’s work through the years is offered.
#nasf
Featured Content
Immersion Deposit Pretreatments for Electroplating on Aluminum
Part 1: A Literature Review
by
David S. Lashmore
Editor's Note: Originally published as D.S. Lashmore, Plating and Surface Finishing, 65 (4), 44-47 (1978), this paper was the first of several reports on AES Research Project #41, a study of the technology of plating on aluminum, at the National Bureau of Standards, now the National Institute for Science and Technology. A printable version of this paper can be accessed HERE.
ABSTRACT
A review of immersion pretreatments for the plating on aluminum is presented with an emphasis on those papers representing major innovations in the technology. The zincate pretreatments seem to dominate the literature; however, recent innovations have been reported which offer both improved adhesion and corrosion performance. In spite of the many processes available, serious problems exist both in alloy sensitivity and in scaling the process up for industrial production. Fundamental work seems to be required in determining the role of the microstructure and alloy composition on adhesion performance.
Introduction
The interest in the plating of aluminum for its decorative and physical properties has greatly increased in recent years. The high strength to weight ratio of aluminum allows a substantial savings in weight for most applications. This is particularly important in the automotive industry and most likely will become more important in other areas when it is realized that plated aluminum composites can replace heavier (and perhaps more expensive) materials and at the same time offer equivalent durability and corrosion resistance.
This review is intended to update the literature dealing with immersion deposit pretreatments for the plating on aluminum. Excellent reviews of some of the early work, especially of the zincate type of process, are available,1-5 so only those early papers are considered which seem to represent major advances in the technology.
Electroplating directly on aluminum is made extremely difficult by the naturally occurring oxide which seems to inhibit adhesion. Anodic type pretreatments (methods to produce a thick, porous, artificial oxide into which the electroplated metal can lock or key) are not considered here. All of the successful techniques reported below incorporate some method of removing the natural oxide and subsequently plating a metal either directly onto the aluminum or onto the previously formed immersion film.
The zincate process
The first record of a zinc immersion process for the plating on aluminum was a 1927 patent by Hewitson.6 Modifications of this process were made by Korpium in 1939.7 These include: the development of the double zincate procedure, the addition of copper to the zincate bath; and the procedure whereby copper is deposited from a copper cyanide solution containing B2O3 following the zinc immersion step. The double zincate process consists of dissolving a first zinc immersion film in a solution of 50% nitric acid and then repeating the immersion step. The much thinner zinc film which forms on the second dip provides improved adhesion, uniformity and better corrosion resistance.
The zincate process consists of (a) a solvent degreasing step, (b) a mild alkaline etch cleaning step followed by (c) a dip in 50% nitric acid to remove the smut, then (d) the zinc immersion dip. and, usually, (e) copper plating from a cyanide solution, for those alloys whose surfaces are covered by a heavy oxide, such as those in the "T" temper, a deoxidizing etch is required before the alkaline etch. The double zincate repeats steps (c) through (d). The effects of the etching procedure have been investigated in some detail by Bullough and Gardam.1 Their work seems to indicate that the effect of the etchant is to remove all the inclusions from the alloy surface. They claim that this procedure has the important additional benefit of exposing a number of families of crystallographic planes. Bullough and Gardam postulated that good adhesion depends on developing an epitaxial relationship between aluminum and zinc planes exposed by the etching procedure. Following the etching step, an acid desmutting step is usually necessary. The function of the acid is the removal of the reaction products as well as inclusions not dissolved by the etchant, leaving pure aluminum alloy covered by a thin adherent oxide. The fourth step in the zincate process involves the immersion of the aluminum substrate into the zincate solution itself. The aluminum oxide is dissolved by the sodium hydroxide, leaving the bare aluminum to take part in the chemical displacement reaction, whereby three zinc atoms are deposited for every two aluminum atoms which pass into solution. Good adhesion has been obtained using several different bath compositions, suggesting that adhesion may not be sensitive to the exact proportion of ZnO and NaOH.
The effect of etchants on the zinc deposition has also been investigated by Keller and Zelley.2 Their results are in substantial agreement with those of Bullough and Gardam. However, Keller and Zelley suggested that the deposition of the zinc ceases once the aluminum surface is covered. They commented that the heavier zinc deposits tend to be spongy and so give poor adhesion. They noticed a direct correlation between adhesion of the zinc deposited and the corrosion resistance of the plated aluminum composite.
In 1951, Bailey3 published his study on the effects of the solution composition and immersion times on adhesion. His results indicate that concentrated solutions give better adhesion than dilute solutions, with maximum adhesion occurring within a range of 380 to 520 g/L NaOH plus 30 to 70 g/L ZnO for a one min immersion. In 1952. Zelley9 showed that substantial improvements in adhesive strength and corrosion resistance are obtained by the addition of ferric chloride and Rochelle salts to the bath.
Solutions containing fluoride anions
Immersion treatments for aluminum with solutions containing fluorides of metal and additional agents were developed by Perner10 in 1942. Perner's work showed that it is possible to obtain very adherent deposits from solutions containing fluoride. The emphasis of Heiman11 was on zinc, tin and cadmium. Heiman claims to have prepared zinc deposits whose adhesion is greater than the cohesion of the aluminum itself and has proposed that this is due to the solubility of the aluminum oxide in the acid solutions containing the fluoride anion.
Using solutions containing the fluoride anion, Heiman found that deposits formed with zinc gave equal or better adhesion than the comparable zinc deposits formed with solutions made up of zinc oxide and sodium hydroxide. Deposits of tin and cadmium formed by immersion in solutions containing the fluoride anion gave adhesion superior to similar deposits formed using other techniques (unspecified) available at the time.
In spite of the work of Heiman. it seems that the most successful zinc immersion pretreatments, from the standpoint of adhesion, economy and industrial type plating, do not contain fluoride.
A zinc alloy immersion process
A successful variation of the zincate process involves the substitution of zinc sulfate for the zinc oxide and additions such as iron, copper, tin, lead or nickel. It is reported in the literature12,13 that the Bondal process,14 a zinc alloy immersion procedure involving nickel sulfate, zinc sulfate, sodium hydroxide, potassium hydrogen tartrate, copper sulfate and ferric chloride, is capable of producing a zinc alloy immersion deposit whose adhesion and corrosion resistance by far exceeds any of the other above mentioned zinc immersion treatments.12 In particular, a comparison of peel strengths, as shown in Table 1, indicates that by using the zinc alloy (or Bondal) process, more than an order of magnitude improvement over the standard zincate (50 g/L zinc oxide, 262 g/L sodium hydroxide) may be expected. However, it must be noted that a comparison of the Bondal process with the more commonly used double zincate (with ferric chloride and Rochelle salts) was not included in this study.
Table 1 - Adhesion of nickel to aluminum prepared by different methods.
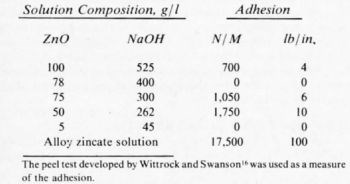
The zinc immersion film produced by this process consists of 86% zinc, 8% copper and 6% nickel. It was shown by Wyszinski13 that the function of the copper and the nickel was to slow down the rate of the alloy formation and to inhibit any possibility of a continuing chemical reaction on the aluminum surface. Such and Wyszinski12 have claimed that the extremely fine grain size of the immersion deposit coupled with the small amount of material on the surface available for analysis has made quantitative evaluation of the film structure difficult. They have also found an adhesion dependence on the precipitation (aging) treatment to which the aluminum alloy is subjected. Excellent adhesion 450 N / 25 mm (100 lb./ lineal in.), was found in a solution heat treated casting alloy LM8 (AA356), but a marked decrease, i.e., 115 N / 25 mm (26 lb./ lineal in.), in the same alloy after a precipitation treatment. They found adhesion in the as-cast alloy to be 350 N / 25 mm (80 lb./ lineal in.), which was comparable to the solution heat treated alloy, even though a great difference in the microstructure exists. They speculated that this difference in adhesion was due to the coherent precipitates formed and to their subsequent effect on the local potentials of the aluminum alloy surface.
Biased ac plated brass
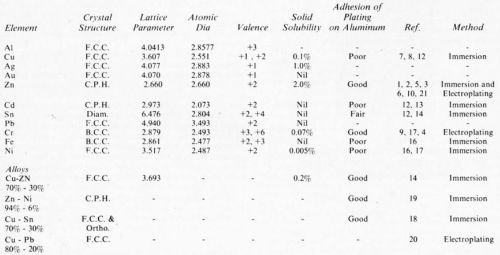
A non-immersion treatment for the plating of aluminum was recently reported in the literature by Schwartz and Newkirk.16 This procedure was developed on the basis of Hume-Rothery alloy theory.17 It was assumed that optimum adhesion of electrodeposits to a base metal is obtained when the deposit is formed on a clean oxide-free surface and when "the deposited metal" is of a comparable crystal structure to permit crystallographic coherence and when extensive solid solution alloying occurs, temperature permitting. As shown in Table 2 reproduced from Schwartz and Newkirk. the system Cu-Zn shows some promise for providing good adhesion based on a presumably similar crystal structure and a lattice parameter which is about 10% less than that of aluminum.
Table 2 - Metal systems for possible direct deposition on aluminum.
It was thought that by employing a biased AC voltage, the essential caustic solution would be aided in breaking down the aluminum oxide during the positive cycle and that plating would take place during the negative cycle.16 Adhesion testing of the resultant plated alloys (pure aluminum and 6061-T6) was accomplished by first plating the alloy with copper and then with tin. The tin was then used as a solder to attach the specimen for adhesion testing. Similar specimens were prepared using the zincate technique. A comparison of the results of these two procedures is shown in Table 3.
Table 3 - A comparison between adhesion of two different pretreatments for plating aluminum.

It was necessary to heat both of the above specimens to 260°C to melt the tin and subsequently form the solder bond. This heat treatment makes somewhat difficult the adhesion comparison of specimens prepared by these procedures with measurements made by other techniques at room temperature (note also that the values listed are shear stress measurements). Even though this technique seems quite promising, difficulties were encountered in scaling the process up for commercial development.18
Stannate pretreatments
A detailed description of the stannate pretreatment is available in the literature.19-25 The pretreatment may be either electrolytic or of the immersion type, the latter being quite similar to the zincate pretreatment discussed earlier. A major difference between the zincate and stannate processes, in operation, is that the article to be plated is not rinsed following the stannate treatment, but rather is transferred wet from the stannate bath and into an electrolytic bronze bath with voltage applied. Apparently some tin is deposited during the immersion process;26 however, it would seem that the function of the stannate dip is to first remove the oxide normally covering the aluminum (to activate the surface) and then to somehow protect the bare metal as the part is subsequently transferred into the bronze bath.
For some applications, the stannate type pretreatment seems to be the currently preferred process when the article to be plated is to be subjected to a corrosive environment. Its "corrosion resistance" has been demonstrated27 to be far superior to that of the zincate process, exhibiting very little of the undercutting type of corrosion which seems to characterize the latter; however, the stannate process seems to be somewhat sensitive to alloy type.
Alcoa 661 process
A zincate pretreatment process has been developed which differs in a significant way from all former processes.28,29 The function of the zincate immersion step in this process is to first remove the aluminum oxide and then to coat the article to be plated with zinc. This zinc coating is then dissolved in the next bath, usually nickel, leaving the article with a metallic (nickel) film in direct contact with the bare, mostly oxide-free aluminum. The dissolution of the zinc is monitored by measuring the electrode potential difference and the plating process started after a predetermined voltage has been reached.
The corrosion performance of this still experimental process has been shown to be quite good, as is the adhesion of the nickel to the aluminum substrate. This process has the additional advantage of being free of cyanides.
Other related pretreatments
- A nickel immersion process had been developed in 1928 by Work.30 However, it seems that the adhesion of subsequent coatings was dependent on a mechanical keying action into the very rough surface that resulted from his procedure.
- Work31 has developed, mainly for corrosion protection, a process for plating zinc directly on aluminum.
- Work and Slunder32 have developed a procedure for depositing chromium directly onto aluminum from a chromic acid solution containing sulfuric acid.
- A direct nickel plating bath (using a solution containing nickel fluorides, nickel chloride, boric acid and nickel fluoborate) has been described by Atkinson33 but does not seem to be under commercial development.
- The Vogt process is a direct zinc plating procedure involving a complex etching pretreatment followed by electroplating from a caustic zinc solution containing chloride ions and cyanide. A thin layer of brass is electroplated on the zinc, followed by nickel. Using this process, a baking treatment is necessary for good adhesion.
- A method of direct plating of copper onto aluminum wire is described by Janjua, et al. (1973).34
- The zincate process usually implies that a copper strike from a tartrate type solution is electroplated onto the zinc coated aluminum; however, it is also possible to use a neutral nickel strike35 or a nickel glycolate strike36 instead of the copper. Both of these proprietary nickel baths have the advantage of being free of cyanide and have demonstrated good adhesion. In a corrosive environment the neutral strike has been shown to be an improvement over the copper strike,37 even though no data seems to be available on the glycolate strike, it should offer comparable performance.
Discussion and conclusions*
A number of immersion type pretreatments are available for the plating of aluminum. The mechanisms for the more successful of these processes are reported to be quite similar, involving both a removal of the oxide and protection of the bare metal until such time as a metallic layer can be deposited.
Though a number of techniques are available for the plating of aluminum, a great many problems still remain. The widely used double zincate has been shown to be quite susceptible to undercutting corrosion when subjected to a severe environment. However, the zincate type treatments are quite amenable to a variety of alloys and have demonstrated good adhesion in a non-corrosive environment. This seems to be particularly true of the zinc alloy process known as the Bondal process.
The stannate type of pretreatment is now being used commercially and seems to be quite successful in a corrosive environment but has shown selectivity for alloy type. For example, the transfer time between steps depends upon alloy type. In addition, the stannate treatments now require a bronze strike from a cyanide bath, which provides some problems with waste disposal. A number of processes, such as the biased AC plated brass, have proven to be successful on a small scale, but have posed some problems when they arc scaled up for production. The A661 process seems to be quite promising on a small scale and should offer no special problems upon scaling up for production, since the baths have been in industrial use for some time. However, further testing seems to be indicated, as zinc contamination of the nickel bath may be a problem.
There seems to be no consensus among the authors quoted above concerning the mechanisms of adhesion of deposited metallic layers to aluminum. It was proposed by Bullough and Gardam1 that crystallographic coherence (or epitaxy) was a requirement. Whereas epitaxial electrodeposited films are found under certain conditions and have even been observed in the zinc-aluminum system,39 little evidence is presented to support the theory that epitaxy over a large area is an adhesion requirement. The opposite case seems to be true: that is, x-ray and electron diffraction studies by Bailey3 indicate that there is no continuity of structure over extensive areas between the aluminum and the zinc. This is supported at least in the case of 99.99% aluminum by work at this laboratory39. There does, however, seem to be a correlation between the porosity of the deposited layer, in the case of zinc, and the adhesion of subsequently plated layers. It has also been shown that thick zinc immersion deposits on aluminum are porous, thereby accounting for the poor adhesion of the thick deposits.
Hume-Rothery alloy theory has been extended to electrodeposited films16 with good adhesion, according to the theory, depending upon the dual requirement of similar crystal structure and good solid solubility. Though this theory seems to have led to a successful plating couple, i.e., brass-aluminum, some glaring exceptions exist. For example, excellent adhesion of nickel has been demonstrated on aluminum,29 even though its solid (bulk) solubility in aluminum is only 0.005% (see Table 2) and its lattice parameter differs significantly from that of aluminum (4.0413 Å for aluminum and 3.517 Å for nickel). Tin is another example of this type. Diffusion of the deposited films into the aluminum seems to have been neglected by most authors. Though diffusion into the bulk material is in most cases negligible in the temperatures under consideration, diffusion along grain boundaries is certainly not and is consistent with the evidence that adhesion increases with temperature. In any case, very few publications on the plating of aluminum deal with the more fundamental aspects of either adhesion or the deposition process itself. Further work seems to be required in developing an understanding of the mechanism(s) of adhesion and its relationship to the aluminum alloy composition and microstructure.
Acknowledgment
I would like to thank both the Aluminum Association and the American Electroplaters' Society for their financial support. Thanks are especially due to Hank Wittrock of Kaiser Aluminum for furnishing an excellent bibliography on the plating of aluminum.
References
1. W. Bullough and G. E. Gardam, J. Electrodepositors Technical Society, 22, 169 (1947).
2. F. Keller and W.G. Zellev, J. Electrochem. Soc., 97, 143 (1950).
3. G.L. Bailev, J. Electrodepositors Technical Society, 27, 233 (1951).
4. R.V. Vanden Berg. Aluminum, Ed. bv K.R. Van Horn, Vol. III, 685, ASTM publication. Metals Park, OH (1967).
5. S. Wernick and R. Pinner, Finishing of Aluminum, Robert Draper Ltd., Great Britain (1956).
6. E.H. Hewitson, U.S. Patent 1,627,900. (1927).
7. J. Korpium, U.S. Patent 2,142,564 (1939).
8. ASTM Recommended Practice B253-73, "The Preparation of the Electroplating on Aluminum Alloys by the Zincate Process." (Note: this practice is currently being updated to include commercial pretreatments).
9. W.G. Zelley, J. Electrochem. Soc., 100 (7), 328 (1953); also see U.S. Patents 2,650,886 (1953) and 2,676,916 (1954).
10. L. Perner, U.S. Patent 2,297,241 (1942).
11. S. Heiman, J. Electrochem. Soc., 95 (5), 205 (1949).
12. T.E. Such and A.E. Wysznyski, Plating, 52 (10), 1027 (1965).
13. A.E. Wvszvnski, Trans. Inst. Metal Finishing, 45 (4), 147 (1967).
14. R. Leloup, British Patent No. 1,007,252 (1965).
15. H.J. Wittrock and L. Swanson, Plating, 49 (8), 880 (1962).
16. B.C. Schwartz and J.B. Newkirk, Plating, 59 (5), 431 (1972).
17. W. Hume-Rothery. Electrons, Atoms, Metals and Alloys, 3rd Ed., Dover Publications, New York (1963).
18. B.C. Schwartz, personal communication.
19. E.J. Seyb, J.C. Jongkind and L.P. Gowman. Proc. Amer. Electroplaters’ Soc., 51, 133 (1964).
20. J.C. Jongkind. Trans. Inst. Metal Finishing, 45 (4), 155 (1967).
21. J.C. Jongkind. Plating and Surface Finishing, 62 (12), 1135 (1975).
22. J.C. Jongkind and L.P. Gowman, French Patent 1,398,605 (1965).
23. J.C. Jongkind and P.G. Kenedi, U.S. Patent 3,274,021 (1966) .
24. L.P. Gowman and J.C. Jongkind, Canadian Patent 811,131 (1969).
25. J.C. Jongkind and L.P. Gowman, German Patent 1,496,899 (1964).
26. J.R. DePew. "Activation of Aluminum for Electroplating," internal report of M&T Chemicals Inc.
27. G.A. Dibari, Plating and Surface Finishing, 64 (5), 68 (1977).
28. "ALCOA 661 Process Electroplating Pretreatment for Aluminum." Alcoa internal report. (June 22. 1976).
29. W.P. Kampert, U.S. Patent 3,989,606 (1976).
30. H.K. Work, Trans. Electrochem. Soc., 53, 361 (1928).
31. H.K. Work, Trans. Electrochem. Soc., 60, 117 (1931).
32. H.K. Work and C.J. Slunder, Trans. Electrochem. Soc., 59, 429 (1931).
33. Atkinson, Canadian Patent 590,840 (1960).
34. M.B. Janjua, et al., Plating, 60 (11), 1124 (1973).
35. M.L Baig, U.S. Patent 3,417,005 (1968).
36. L. Missel, Plating and Surface Finishing, 64 (7), 32 (1977).
37. G.A. DiBari. Plating and Surface Finishing, 64 (5), 68 (1977).
38. O.J. Klingenmaier and S.M. Dobrash, "GM Research Publication GMR-2266," (Oct. 13. 1976).
39. D.S. Lashmore. (to be published).
About the author (at time of publication)

Dr. David S. Lashmore received his Ph.D. in Materials Science from the University of Virginia in June 1977 and is currently Technical Director of AES Research Project 41, "Plating on Aluminum." He obtained a B.S. degree in engineering science and mechanics from the University of Florida in 1968, and a M.S. degree in physics from Michigan Technological University in 1970. He was the recipient of a National Science Foundation Undergraduate Research Grant in 1968 and was awarded the Thornton Fellowship for 1975-76 by the University of Virginia. He has worked as a physics instructor and in other capacities and has a number of publications. He is a member of Sigma XI.
Accomplishments since time of publication
(1977-1993): David jointed NIST-Gaithersburg and led the electrodeposition group for 17 years. He developed processes to electrochemically synthesize artificial superlattices. This research led to the discovery of giant magneto resistance in electrochemically derived structures. He developed a coated powder technology now used for soft magnetic materials and mercury-free dental technologies. He also worked on fast diffusion processes to create intermetallic alloys at low temperatures. He was awarded the Department of Commerce Bronze Metal, the Electrochemical Society Electrodeposition Division Research Award, the AESF William Blum Scientific Achievement Award and was ECS Electrodeposition Division president.
(1993-2002): He co-founded Materials Innovation where he was CTO for nine years and helped develop: (1) a highly uniform coated steel P/M alloy, and (2) a new kind of phosphate coated P/M use to create soft-magnetic iron powder for magnetic parts made by powder metallurgy. This material won the Powder Metallurgical Society’s award for the invention of the year. He also co-invented a powder compaction press and powder feed system that won the Time Magazine Invention of the year.
(2002-2013): He joined Synergy Innovations from which Nanocomp was spun out in 2004. Nanocomp Corp. is now a Huntsman company. He was the first to create large 4’ by 8’ sheets of pure carbon nanotubes (CNT). Some of this material was used on the Juno Satellite.
(2013-present): He joined the University of New Hampshire with efforts focused on, (1) synthesis of continuous boron nitride nanotube (BNNT) tapes and yarns, and (2) improved the electrical conductivity of CNT fiber, high-performance batteries, and multi-junction solar cells. Programs are underway on the synthesis of artificial superlattices, batteries and improvements of electrical contacts. He is an adjunct Prof. of ME, at the University of Cincinnati.
(2015- present): David Lashmore and Pavel Bystricky formed American Boronite Corporation to create boron nitride nanotubes (BNNT) based on a scaled-up CVD synthesis process. Boronite Corporation focuses on the large scale synthesis of boron nitride nanotube structures, CNT-based continuous quantum (chirality-controlled**) wires and their derivative products. (see www.boronite.com). The company is located near Boston, MA.
* Endorsement any pretreatment process is not intended by the author.
**An object or system is chiral if it is distinguishable from its mirror image.
RELATED CONTENT
-
AES Research Project #41: Part 4: Adhesion Failure of Electrodeposited Coatings on Anodized Aluminum Alloys
An SEM study of peel-test adhesion specimens from plated coatings on anodized aluminum shows that failure can be categorized in three different modes: (1) specimens exhibiting poor adhesion strength, which fail at the anodic film/coating interface; (2) specimens with good adhesion strength, which fail by local fracture of the anodic film and (3) specimens with excellent adhesion strength , which fail when the applied load is greater than the strength of the alloy substrate. The effect of anodizing parameters and alloy composition on peel test failure are discussed.
-
Hybrid Sol-Gel Coatings in Surface Engineering
A look at the use of modified sol-gel polymer films and hybrid system coatings, as well as the methodologies for evaluating the mechanical properties of the coatings.
-
A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .


















