Anodizing that Withstands H2O2 Cleaning
Testing color anodized finishes in hydrogen peroxide gas plasma sterilization.
Color anodized aluminum is widely used as a material in the medical device industry. From tool handles to trays, these repeat-use devices undergo regular cleaning and sterilization treatments and it is vital that the original finish remain preserved throughout.
Several types of sterilization methods are in use today, but those that incorporate hydrogen peroxide injection are particularly challenging for a color anodized finish to endure, since fading or significant discoloration typically occurs after only a few sterilization cycles.
Featured Content
Here, we explore various coloring and sealing treatments exposed to one such method of sterilization: Sterrad. Manufactured by Advanced Sterilization Products in Ervine, Calif., it’s a system that uses low-temperature hydrogen peroxide gas plasma technology to sterilize instruments and medical devices. Which treatments for anodized aluminum can hold up to multiple rounds of Sterrad?
Coloring Technologies
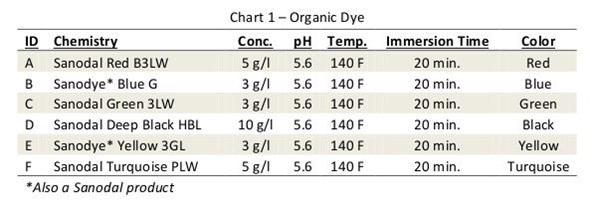
Anodized aluminum can be colored using a variety of technologies that can loosely be divided into two classes: organic and inorganic.
Organic colorants, or dyes, are by far the most widely used for decorative finishes. The porous anodized aluminum is simply dipped in a dye bath, and over time, dye is absorbed into the surface. The color range is virtually endless as dyes are used at any number of depths of shade and in any combination.
Dyes tend to be large organic molecules that may contain a metal ion. Every dye is unique and has its own fastness characteristics, but all fade to some degree as a result of bond cleavage within the organic molecule. This cleavage can occur as a result of UV irradiation, chemical oxidation or a combination of both.
The dyes chosen for this study were taken from Clariant’s Sanodal range of super high fastness products, dyes that have been developed, tested and proven durable enough for extended outdoor use.
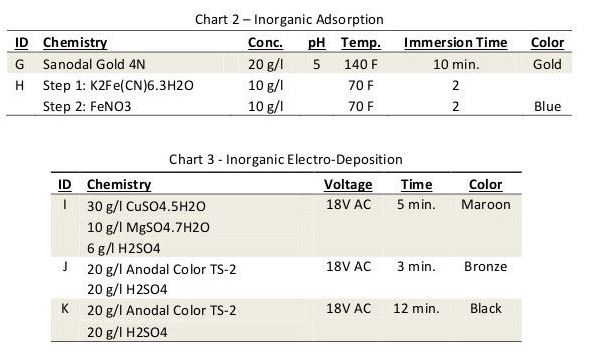
Two common inorganic coloring techniques used to provide decorative finishes on anodized aluminum include adsorption and electro-deposition of metal-containing pigments. The most common adsorption method is used to create a gold finish by dipping the anodized aluminum in an ammonium or sodium ferrioxalate salt solution.
The heavy metal salt is hydrolyzed and an iron-based pigment is precipitated within the pores of the coating. A lesser known but similar technique uses a sequential dip process where first the part is dipped in a solution of potassium ferrocyanide and then in a ferric nitrate bath. The ensuing double decomposition treatment results in the creation of a nontoxic blue pigment commonly referred to as Prussian blue.
Electro-deposition of metallic pigments is achieved by immersing the anodized aluminum in a metal salt solution and applying an AC potential. During the cathodic half cycle, particles of metal are deposited at the bottom of the pores. During the anodic half cycle, the ultra-thin barrier layer oxide separating the porous coating from the aluminum is given time to stabilize.
Over time, the metal particles fill the pores and the resulting color deepens. This technique is widely used around the world to produce bronze and black finishes for exterior architectural components. Although limited in color range, some variation can be achieved by varying the coloring time and the metal deposited. In the case of this experiment, tin was used to create a bronze and black finish; copper was used to produce maroon.
Sealing Technologies
It is well known that without a quality seal, one cannot expect a durable, fade-resistant finish. Technologies used for sealing are highly varied. There are boiling seals, mid-temperature seals and room temperature seals, usually referred to as cold seals. Sequential processes, which include duplex and high-barrier seals, have been used for special applications that require enhanced corrosion or alkali resistance.
Boiling seals can be nickel acetate-based or just purified water. Hydration or swelling of the oxide is the primary seal mechanism but if nickel is present, hydration is accompanied by nickel precipitation to further improve the seal quality, and in many cases, the fade resistance of the colorant. Mid-temperature seals work on the same principle, but these products contain surfactants that act as a catalyst, allowing them to run at temperatures of as much as 40°F below the boiling point.
Cold seal chemistries are based on nickel fluoride. The seal mechanism includes the precipitation of nickel compounds with the simultaneous reaction of fluoride with available aluminum. The seal is usually completed by dipping the part in hot water for a few minutes in order to allow it to “cure.” Cold seals produce a chemically resilient finish, and in the case of many dyes, will improve fade resistance.

Duplex seals are often used in the automotive industry due to the enhanced corrosion protection they provide. The standard duplex seal is a sequential process where an initial dip in a hot nickel acetate solution is followed by a boiling water treatment. A variant of this is the little-known Hi-Barrier Dual seal, which exchanges the boiling water treatment for a dip in a hot silicate solution. Silicate seals are known to improve the coating’s alkali resistance.
Unpublished research has shown that substitution of a standard cold seal for the hot nickel acetate step will further enhance alkali resistance beyond that which the silicate dip alone provides. This sequence is referred to as a Modified High Barrier Dual seal. Studies have suggested the suitability of a similar process for producing automotive trim parts to meet the rigorous pH 13.5 test used in that industry.
Experimental
AA6061 sheet stock was used throughout our experiments. Panels were alkaline soak cleaned, etched, deoxidized, and anodized for 45 min. at 15 amps per sq. ft. to create a porous coating measuring 0.7 – 0.8 mil thick. The panels were then colored using the conditions listed in Charts 1-3.
Each pre-cut colored panel was carefully split into strips, and each strip was sealed using conditions listed in Chart 4. In the case of the inorganic colors, only seals 1-7 were used.
Two sections taken from each of the 95 color/seal combinations were submitted to Advanced Sterilization Products to be processed in a Sterrad 100NX Sterilizer for 100 standard cycles. Every 10 cycles, the samples were removed and washed.
A comparative visual assessment of each of the colored finishes before and after undergoing 100 Sterrad sterilization cycles is shown in Chart 5. Panels that survived the treatment without notable fading are identified by a dark green box; panels that completely faded are indicated in red.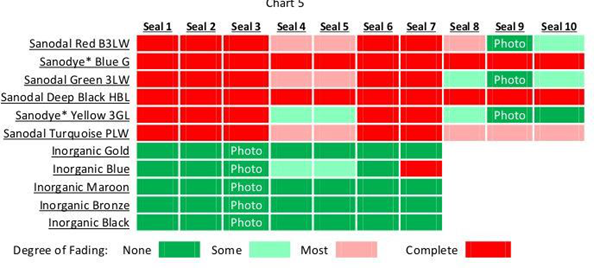
Discussion
An oxidative environment—such as a Sterrad sterilization cycle—can fade anodized coatings that are colored with organic dyes. Multiple cycles can do so catastrophically. It has, however, been demonstrated that dyed coatings can be produced to withstand 100 such cycles with no noticeable effect.
With the correct seal system, Sanodal Red B3LW, Yellow 3GL and Green 3LW are among a list of dyes that can be successfully used for this application. On the other hand, Sanodal Deep Black HBL, Blue G and Turquoise PLW should not be used regardless of the seal system. It is not known why some dyes can and others cannot be used and therefore impossible at this time to predict the applicability of other dyes.
With the exception of the unsealed reference series (No.7), the seal treatments used in this study were all of very high quality. Even so, for the most part they failed to protect the dyes from fading. The MHBD seal (No.9) clearly proved to be the best performer, allowing virtually no color deterioration of the red, green and yellow dyed coatings. As described earlier, this two-step seal process included a standard cold seal followed by a boiling silicate-type seal. In addition to color retention, this seal provides improved alkali resistance, which can be expected to help protect the finish from harsh detergents used to clean the equipment prior to sterilization.
For the most part, this study showed that inorganic colors were unaffected by Sterrad sterilization. The gold, blue, bronze, maroon and black easily pass the visual test for color degradation. Although this is not an altogether surprising result, it was interesting to prove none the less.
With the exception of the Prussian blue, even the color of the unsealed panels were unaffected by the sterilization cycles. This suggests that other inorganic treatments including integral coloration such as hard coat processing, and interference coloration would produce similar results.
Mark Jozefowicz is vice president of Technical Services for Reliant Aluminum Products. He can be reached at mark.jozefowicz@reliantaluminumproducts.com. Thanks to Chris Coker and his colleagues at Advanced Sterilization Products, Division of Ethicon, Inc. a Johnson & Johnson company, for running the lengthy Sterrad test program.
RELATED CONTENT
-
Fixing Corrosion Between Anodized Aluminum and Steel
Anne Deacon Juhl, Ph.D., with AluConsult, says Galvanic corrosion is due to an electrical contact with a more noble metal or a nonmetallic conductor in a conductive environment.
-
Understanding Corrosion and Salt Spray
How it’s produced, NSS testing and how to get the best results possible.
-
Aluminum Anodizing
Types of anodizing, processes, equipment selection and tank construction.


















