Balancing an Electroless Nickel Bath to Optimize Performance
While today’s EN baths are much more user friendly than they were even 10 years ago, plating shops still strive to maintain consistent, trouble-free operation. The following provides a basic understanding of EN bath control.
#masking #pollutioncontrol

Since its discovery in the late 1940s, electroless nickel (EN) has become widespread in worldwide manufacturing. It is used in almost every industry: automotive, oil and gas, aerospace, electronics and consumer goods, to mention just a few. The U.S. market for EN plating today is estimated at over $400 million.
Featured Content
While today’s EN baths are much more user friendly than they were even 10 years ago, plating shops still strive to maintain consistent, trouble-free operation. The following provides a basic understanding of EN bath control.
Bath Stability
EN baths are auto-catalytic, meaning they are self-starting, and do not need an external DC power supply or rectifier to coat a substrate. The “electricity,” or electrons, come from the bath itself; specifically from the reducing agent sodium hypophosphite. Because the power for the nickel deposition comes from a chemical in the bath (sodium hypophosphite), and because most chemical reactions are affected by temperature, pH, concentration, contamination and the presence of other chemicals, the EN deposition reaction can vary widely depending on how the bath chemistry and operating parameters are controlled.
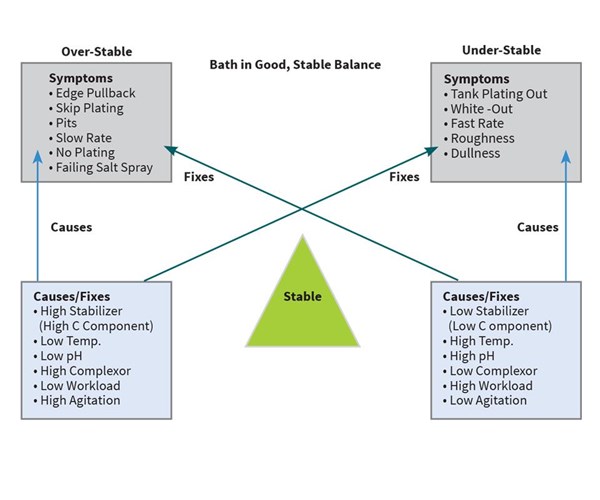
When the EN bath and its parameters are in an optimum state, the reaction and the deposition goes smoothly, and a quality EN deposit of the proper phosphorus content with minimal pitting or roughness is achieved. But, if not controlled properly, numerous issues can surface. Most of the issues relating to bath imbalance can be characterized as the bath being either too stable or not stable enough. By understanding the concept of bath stability, anyone can operate a fairly trouble-free EN system.
Figure 1 shows a “balance” that is representative of most EN baths. On one side of the balance is “over stability,” on the other side of the balance is “under stability.” When balanced by correct pH, temperature, concentrations, bath age, loading and agitation, a trouble-free operation can be achieved. However, if one or more of these parameters goes out of range, the bath can become either over stable or under stable, and one or more of the symptoms outlined in the diagram can occur.
By understanding EN bath stability and this diagram, any of the symptoms on one side of the balance can be relieved by implementing one or more of the fixes on the opposite side of the balance. A few examples follow:
Bath Plate-Out
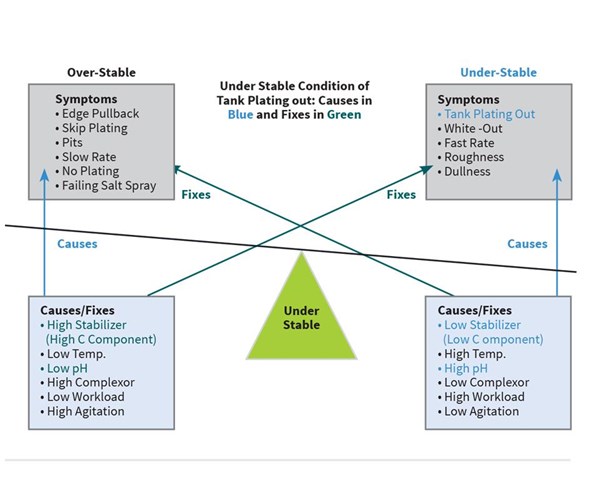
The mid-phos EN bath at Plating Shop A is at two metal turnovers, and starts to plate-out on the tank walls at the beginning of the shift on Monday morning. Because they have several hot jobs that need to get out today, they can’t afford to pump the tank out to passivate it. And even if they could, how can they be sure it won’t plate out again?
Looking at Figure 2 on page 16, they find that tank plating out can be caused by low stabilizer, high temperature, high pH, low complexor (B component), low or no agitation, or too high workload. They perform a hypo titration to analyze for the C component and find that it is down to 75 percent. They find the pH a little high at 5.2. They quickly make the appropriate adds of C component and lower the pH to 4.9 which slows the plate-out and assures that after pump out and proper passivation that night, the bath will not plate out the next day.
Edge Pullback and Skip Plating
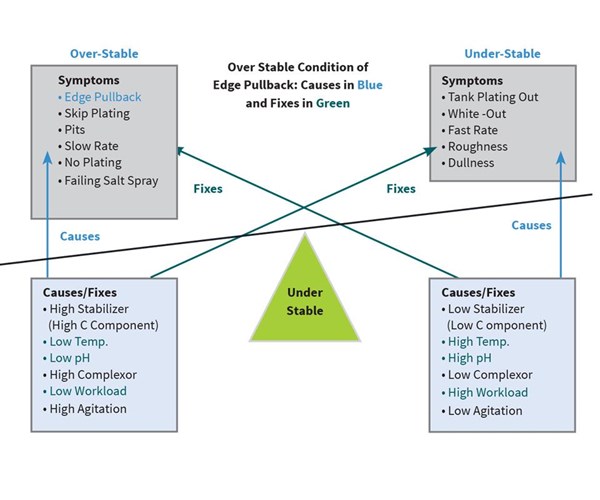
Two weeks later at the same plating shop, a worker pulls a part from the high-phos EN and notices that there appears to be little to no plating along the outside edges of the part. Perplexed, he cleans and puts another of the same part into the bath. When he pulls this second part, it still has the appearance of no plating along the edges.
He alerts his boss, who knows that this is a classic case of edge pullback. Looking at the diagram (Figure 3), they determine that edge pullback is a symptom of the bath being too stable and can be caused by one or more of the following: low pH, low temperature, low loading, too high stabilizer (C component), high complexor (B component) or too much agitation.
After examining the bath and the way the worker ran the parts, it was determined that he put only one of each part into the bath at a time. Since the parts were very small (less than 0.5 square feet of area), and the bath volume was 900 gallons, the worker was severely underloading the bath. It was also discovered that the pH and temperature were down slightly at 4.6 and 188°F, respectively. They quickly raised the pH and temperature and loaded 20 parts at a time into the bath. That load plated with no edge pullback.
Small Workload
It is now a few months later at Plating Shop A, and they now understand EN bath stability fairly well. So, when the worker is presented with one very small part to plate, and he has no other work to put in the bath, he knows exactly what to do.
Since the worker now knows that small workloads can cause over-stability issues like edge pullback and skip plating, he knows he needs to push the bath to the under-stable side. So he turns off the air agitation and raises the pH from 4.8 to 5.0. He plates the one small part, and it comes out fine. By understanding EN bath stability, the worker has averted a potential rework.
Although other plating problems can arise from issues outside the EN bath, including pretreatment and base material, by understanding and controlling the stability of an EN bath, many common problems can be averted, solved or minimized.
Lloyd Ploof is Metal Chem, Inc.’s regional sales manager and technical service provider for the Northeast and Midwest U.S. For information, please visit metalchem-inc.com.
RELATED CONTENT
-
8 Things You Need to Know About Paint Booth Lighting
Global Finishing Solutions has come up with some helpful insights on lighting for paints booths which plays a crucial role in achieving a quality paint job.
-
Cleaning Magnesium
Question: What is the recommended chemical cleaning process and composition prior to electroless nickel plating for magnesium?
-
Corrosion Testing of Automotive Coatings
Exposure to road salts, UV radiation, heat, moisture and chipping from kicked-up road debris can quickly degrade an automotive coating system.


















