Black Chromium Finishing: Beauty and the Beast
Over the past 10 years, there has been commercial development of black chromium deposits from a trivalent chromium electrolyte. This paper will review the deposit characteristics and operational consideration of these similar, but different chromium plating deposits.
#research #surfin
A Synopsis* of a Presentation given at SUR/FIN 2019 (Rosemont, Illinois) by Richard Macary and Dishant Tailor **, Arlington Plating, Palatine, Illinois, USA
ABSTRACT
Black chromium plating has been commercially available for over 50 years. The original black chromium plating is described in Mil Std 14538 which deposits black chromium from a hexavalent chromium electrolyte. Over the past ten years, there has been commercial development of black chromium deposits from a trivalent chromium electrolyte. This paper will review the deposit characteristics and operational consideration of these similar, but different chromium plating deposits.
Featured Content
Introduction
Beauty and the Beast (French: La Belle et la Bête) is a fairy tale written by French novelist Gabrielle-Suzanne Barbot de Villeneuve and published in 1740. Our comparison with this presentation will not be on the fairy tale but rather the differences between the trivalent and hexavalent chromium-based black chromium plating processes.
Figure 1 compares the appearance of the trivalent and hexavalent process solutions. On the left is a chromium sulfate-based trivalent black chromium plating process. The solution color is deep blue, the tank is relatively clean, and the solution is stable. We easily decided that this process would be called the Beauty in this presentation.
On the right is a chromium trioxide-based black chromium plating process. The solution color is reddish brown. During electroplating, hydrogen gas evolves, which can create a hexavalent chromium-containing mist. This mist must be controlled with wetting agents and or push-pull ventilation. The hexavalent black chromium, at least for the rest of this presentation, will be referred to as the beast!

Figure 1 - A Comparison of trivalent and hexavalent black chromium plating processes.
The objective of this work is to evaluate alternative black chromium electroplating deposits and determine the practical use and suitability of these coatings in interior and exterior automotive and commercial applications. The focus is on physical performance characteristics, including corrosion protection, scratch resistance, stain resistance and appearance of both hexavalent and trivalent-based black chromium deposits. The results should serve as a practical guide for selecting the best combinations of plated coatings to achieve expected performance. We also seek to identify how black chromium deposits compare with other black coating alternatives, including PVD, black powder coat and black electroless nickel.
Background
The patents for black chromium plating date back to 1952. The initial patent related to electroplating black chromium, was issued to Gilbert and Buhman.1 Gilbert and Buhman developed a process utilizing aqueous chromic anhydride and acetic acid to achieve a dark gray to black deposit on rifle parts, artillery parts and military equipment at the Rock Island Arsenal.1 Further patents were filed by Westinghouse Electric in 1956 and Kewanee Oil Company (Harshaw Chemical), issued in 1971.2,3 The Harshaw Chemical patent, eventually was transferred through M&T Chemicals, and is now being sold under the Atotech Chemical tradename Econo-chrome BK.
The first patents for trivalent chromium plating date back to early 1970. Gyllenspentz and Renton of Albright and Wilson; as well as Tremmel of OMI (now MacDermid Enthone), were one of the early pioneers to carry out the development of trivalent chromium electroplating baths. Tremmel’s work focused on utilization of thiazole compounds, whereas Gyllenspentz and Renton’s work focused on use of formate, acetate, bromide and ammonium to achieve a commercially viable deposit.4-7 Moreover, newer commercial processing offering chloride or a sulfate-based chemistry are more commonly used now that are easier to control.
Operational Comparison
Table 1 compares the operating parameters of both the chloride and sulfate versions of the trivalent black chromium and the hexavalent black chromium processes.3 The first and most significant operational difference is in the electrolyte. The trivalent black chromium processes utilize a chloride or sulfate metal salt with chromium for the electrolyte, while the hexavalent black chromium uses chromium trioxide (or chromic acid) for the main component. The trivalent baths operate at a pH range of 2-4 while the hexavalent black chromium operates at a pH of less than 1. The operating temperatures are similar in all three solutions.
Table 1 - Operational comparison of chloride Cr(III), sulfate Cr(III) and Cr(VI) processes.
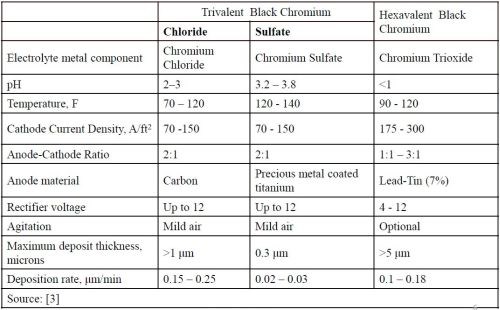
As for throw and coverage, the trivalent black chromium processes have good throwing power and will cover parts more completely without the use of special auxiliary anodes. Standard racking designs can be used.
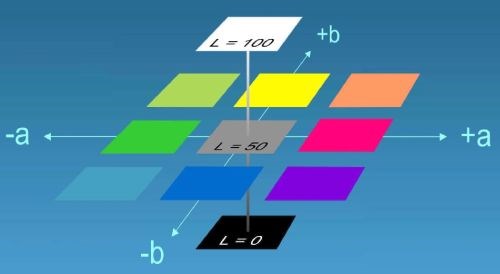
Figure 2 - The concept of L*a*b values in colorimetry.
However, this is not the case with the hexavalent black chromium process. The efficiency is poor leading to a lack of throwing and covering power. Without special rack fixturing (i.e., auxiliary anodes), the black chromium deposit will have multiple shades of gray and gray/black, and several chemical constituents need to be balanced for optimum black over the entire current density range.
How black is black?
In general, color can be measured with a colorimeter using L*a*b values for color and lightness. L indicates the level of lightness/darkness, a specifies redness or greenness, and b specifies yellowness or blueness. The concept is shown in Fig. 2 to describe the lightness and color of a part.
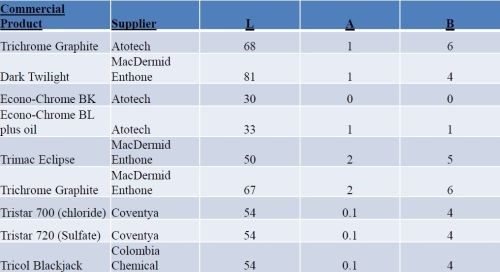
We measured L*a*b data from several black chromium processes and obtained data from various chemical suppliers. The results are shown in Table 2. It should be pointed out that this list of black chromium processes does not represent all the black chromium processes commercially available - these are from the suppliers with whom we have worked. The L values range from zero (jet black) to 100 (white). There are some very low values for color – mostly browns with +a and +b values.
Table 2 - L*a*b values for several commercial black chromium deposits.
Deposit structure
The surface morphologies of hexavalent and trivalent-based black chromium deposits are compared in the SEM photos shown in Fig. 3. The deposit structure is different but both systems deposit hard layers of chromium which provide very good abrasion resistance.
The hexavalent black (Fig. 3a) is more crystalline with a 50/50 composition of chromium oxide and chromium. The deposit exhibits some micro-cracking. It is not uncommon to apply a post treatment with a wax or oil to the hexavalent black finish. With these secondary treatments, you can enhance the visual appearance in many ways.
The sulfate-based trivalent deposit (Fig. 3b) shows an amorphous structure, glass-like, and can get cloudy at higher thicknesses. Chromium sulfate-based deposit is denser than the chromium chloride-based deposit as can be seen in the transmission electron micrographs in Fig. 4. Reports indicate that there is a difference in the ability of the alternate trivalent chromium systems to withstand Russian mud exposure (CaCl2) and this is due to the difference in deposit structure.
Both of these systems can be used in conjunction with the standard automotive exterior multilayer nickel chromium layers.
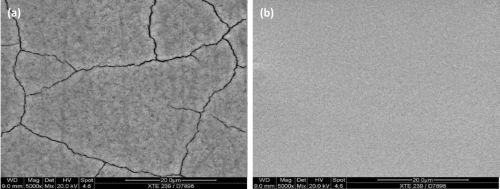
Figure 3 - Surface morphology of black chromium deposits, from (a) hexavalent Cr chemistry and (b) trivalent Cr chemistry (SEM).
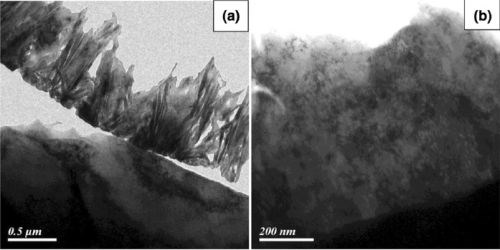
Figure 4 - Deposit structure of trivalent-based black chromium deposits, from (a) a chloride electrolyte and (b) a sulfate-based electrolyte (TEM).5
Environmental health and safety
Hexavalent black chromium uses chromic acid as the main ingredient which is very toxic and should only be used with careful controls. Cr(VI) is classified as a carcinogen and the OSHA Permissible Exposure Limit (PEL) for Cr(VI) is 5 μg/m3. In operation, a respirator and skin protection are required, and the use of a ventilation scrubber system is essential.
Trivalent black chromium is less toxic. Although there is no data available on the carcinogenic potential, the OSHA PEL for Cr (III) is 500 g/m3. Although skin protection is required, with the use of a wetting agent, the system is regulated in a manner similar to nickel plating.
Hexavalent chromium is of significant concern in regard to wastewater treatment. One must first reduce the Cr(VI) to Cr(III) with sodium metabisulfite at low pH. The pH is then raised to 9-10 to form chromium hydroxide, which is precipitated and filtered. Treatment of Cr(III) is similar, but without the first step.
Applications
Black chromium applications are numerous, and include motorcycle, automotive, consumer/medical, industrial applications, as well as in solar panels.
Motorcycles
The trend over the past several years are to style the motorcycles with an increasing amount of black finishing. Figure 5 shows a Harley Davidson Softail CVO (custom vehicles) with “smoked satin chromium” parts with satin black. This coating still requires a satin nickel underlayer. The figure also shows other parts which receive a black chromium finish.

Figure 5 - Examples of black chromium finishes for motorcycle applications: (Clockwise from upper left) Harley Davidson Softail CVO “smoked satin chromium” parts with satin black; black chromium muffler pipes (photo courtesy of Calchromium); black chromium muffler tips (photo courtesy of Polaris Industries); Harley Davidson black chromium master cylinder.
Automotive
Virtually all of the original equipment manufacturers (OEMs), both foreign and domestic, offer black chromium finishes in their offerings. Figure 6 shows some of the styling treatments which use these black finishes.

Figure 6 - Examples of black chromium finishes for automotive applications: (Clockwise from upper left) Cadillac black chromium finish on CTS and ATS models; Honda Accord black chromium grille accent; Honda Accord rear garnish; automotive wheel rims.
Consumer and medical
As shown in Figure 7, consumer and medical applications for black chromium run the gamut from eyeglass frames to medical tools, including the otoscope for examining ears.

Figure 7 - Examples of black chromium finishes for consumer and medical applications.
Industrial / Engineering
Industrial applications are numerous, including the linear motion guide used for positioning in an industrial mechanical device shown in Fig. 8. While color and its uniformity of the finished part is desired, the inherent wear resistance of the black chromium finish is also important.

Figure 8 - Examples of black chromium finishes for industrial applications.
Solar
Black chromium finishes for the solar thermal collectors shown in Fig. 9 has been in use for decades, but economies of scale have led to its increasing use today. The black chromium finish selectively absorbs and retains the solar wavelengths in the visual and infrared spectrum for maximum absorbance.
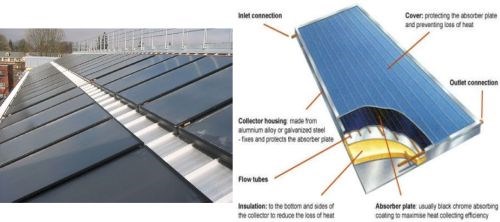
Figure 9 - Black chromium finishes as used on solar thermal collectors (L) and the details of their operation (R).
Alternatives to black chromium
While trivalent black chromium processes offer an alternative to the environmental and health problems associated with hexavalent chromium process chemistry, there are other technologies commercialized or under development which also offer replacements for hexavalent black chromium. Among them are:
- Black anodizing
- Black electroless nickel
- Vapor phase deposition (PVD)
- Black powder coating
- Black paint
- Black oxide
- Black chromate on zinc
- Moly black electrolytic nickel
Summary
- The black color is more prominent today on motorcycles and automotive exterior trim.
- Hexavalent black chromium will provide the deepest black finish.
- Hexavalent black chromium will have the same OSHA exposure, operational, and environmental concerns as hexavalent bright chromium.
- New trivalent black chromiums are darker and the color can be controlled with additives and filtration.
- Trivalent black chromiums will operate similar to a Watts nickel process with less environmental, health and safety concerns.
References
1. L.O. Gilbert & C.C. Buhman, “Black Chromium Plating,” U.S. Patent 2,623,847 (December 30, 1952).
2. K.S. Willson, “Process of Black Chromium Plating, U.S. Patent 3,602,935 (November 16, 1971).
3. D.L. Snyder, “Decorative Chromium Plating,” Metal Finishing, 109 (11A), 177-187 (2011)
4. J. Gyllenspetz and S. Renton, “Trivalent Chromium Electroplating Baths and Electroplating Therefrom,” U.S. Patent 3,954,574 (May 4, 1976).
5. C. Sheu, et. al., “Electrodeposition of Black Chromium-Cobalt Alloy-Based on Trivalent Sulfate Electrolyte,” Journal of Taiwan Institute of Chemical Engineers, 59, 496-505 (February 2016).
6. R. Tremmel, “Trivalent Chromium Electroplating Baths and Processes using Thiazole Addition Agents,” U.S. Patent 4,432,843 (July 29, 1982).
7. Agency of Toxic Substance and Disease Registry - Case Studies in Environmental Medicine (CSEM), (2008); https://www.atsdr.cdc.gov/csem/csem.html.
About the Authors

Richard Macary is the President at Arlington Plating Company and has been with the company for 20-years. Rich has also held positions as Sales Manager, Product Manager, Sales Engineer and Lab Technician working for Enthone, Atotech and Winchester Electronics. Rich attended Waterbury State Technical College with a concentration in Chemical Engineering and graduated with a B.S. degree from Charter Oak College and an MBA from Keller Graduate School. Rich is also a Certified Electroplater Finisher and holds a patent for plating on magnesium.

Dishant Tailor is the Operations Manager at Arlington Plating Company. Dishant has been in the electroplating industry for nine years. His time in the metal finishing industry has been split between Guardian West (part of Flex-n-Gate Corporation) and Arlington Plating Company. During his tenure in the industry, he has worked in various capacities including Process Engineer, Lab Manager, Director of Quality and Operations Manager. Dishant holds B.S. and M.S. degrees in chemistry from Illinois State University, and was part of Product Finishing’s 2018 class of “40 Under 40.”
Footnotes:
*Compiled by Dr. James H. Lindsay, Technical Editor - NASF
**Corresponding authors:
Richard Macary, President
Dishant Tailor, Operations Manager
Arlington Plating Company
600 S Vermont St.
Palatine, IL 60067
Phone: (847) 359-1490
Email: rmacary@arlingtonplating.com; dtailor@arlingtonplating.com
RELATED CONTENT
-
A Pulse/Pulse Reverse Electrolytic Approach to Electropolishing and Through-Mask Electroetching
Research at the authors’ laboratories has focused on pulse/pulse reverse electrolysis on cathodic processes, such as hard chromium plating from non-hexavalent chemistries. This papers describes studies into pulse/pulse reverse electrolysis as applied to electrochemical metal removal processes, such as electropolishing and electroetching.
-
Identifying New Alternatives for Chromium Plating
A comprehensive review of chromium plating alternatives and a new look at a long-forgotten but potentially important process - iron plating
-
Aluminum Surface Finishing Corrosion Causes and Troubleshooting
In this paper, a review of several process solutions, examining coolants, solvent cleaning, alkaline clean/etch and deoxidizing/desmutting, listing intended and unintended chemical reactions along with possible mechanisms that would favor corrosion formation.


















