Controlling Electroless Nickel Baths
Every plating process benefits from process control...
Every plating process requires and benefits from process control. This is especially important when working with electroless nickel (EN) systems, since control is vital to achieve the desired properties. The most common performance criteria are based on specific deposit properties. Most commonly desired deposit properties include corrosion resistance, hardness and wear resistance.
Achieving desired performance standards depends on whether the proper EN system was originally chosen. Also, correctly operating that system dramatically affects the final deposit quality. All EN systems are similar in this respect. The chemistry, operating parameters, surface preparation and equipment considerations are the primary variables that influence deposition rate, coverage, adhesion, smoothness, uniformity and brightness.
Featured Content
Maintaining an EN solution begins with an understanding of important chemical and physical process variables that influence the solution's performance. These variables directly affect the final deposit quality. The three primary areas include the chemical balance, the interaction of rate and loading effects and the influence of the process equipment on the final quality.
Chemistry and chemical balance. Anyone can make up an EN solution, but successful plating only occurs if the solution is properly maintained. The proprietary systems available today are easy to make up and maintain. There are also standard formulations that allow the user to make up his own system.1, 2 Initially, those who make up home-brew solutions have good success producing acceptable deposits. The challenge is to maintain that level of quality throughout the bath life. Self-prepared solutions often have reduced bath life and are routinely waste treated at one solution turnover or less. The key to a successful EN system is proper replenishment. The chemical balance of any EN bath is critical. Neglecting replenishment schedules or maintenance of solution levels can cause non-uniform deposits, premature bath decomposition, slow deposition rates, poor adhesion, poor brightness, pitting or roughness. Usually, the effects of poor bath control are seen immediately after the parts have been plated. Often, the results of poor bath control are seen in substrate corrosion after the parts have been shipped to the end user.
Table I shows the primary components that make up a typical EN system. The balance and control of these constituents are required for a successful operation. Control can become more difficult due to unusual circumstances found in every process line.
| TABLE I—Typical EN Bath Components | |
| Component | Function |
| Nickel Salts Sulfate, Sulfamate, Chloride |
Source of nickel |
| Reducer Hypophosphite Boranes (DMAB) |
Reaction energy source for nickel to be available for reaction. "The Chemical Rectifier". |
| Chelators/complexors Citric acid, Lactic acid, Malic acid |
Allows controlled amount of nickel to be available for reaction. Avoids a runaway reaction. |
| Buffers Borate, Acetate, Succinate |
Inhibits dramatic pH changes |
| pH regulators Ammonium hydroxide Potassium carbonate Sulfuric acid |
To adjust and maintain the operating pH |
| Stabilizers Metallic - Pb, Sn, Mo Organic - S Compounds |
Plating rate control and prevent uncontrolled plate out |
| Wetting Agents bubble release and reduces pitting |
Controls solution's surface tension and H2 |
With a variety of chemical materials comprising these systems, an imbalance will likely cause some negative result. Proprietary systems are usually balanced to maintain the proper chemical ratios if replenishment schedules are followed. Some proprietary systems are easier to operate because of variations in the chemical mix in the replenishments. Some slight modifications of these replenishment systems are common, depending upon specific circumstances related to a particular process line. For example, in barrel EN plating applications, the makeup component containing primarily complexor materials should be added back to the solution during replenishment to compensate for dragout losses. A reduction in the quality of deposit brightness, smoothness obtained or solution life usually occurs if the addition is not performed. The EN chemistry supplier should be consulted for specific replenishment recommendations.
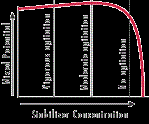
Stabilizers. EN systems have small, ideal concentration levels and tolerances for some of the chemical they use. Stabilizers and brighteners are a good example of these, since they are usually found in the mg/liter range. As shown in Figure 1, stabilizers are important in regulating the plating rate while also preventing the solution from spontaneously decomposing. Keeping the solution level at a nickel concentration above 80% nickel activity is important, because the stabilizer additives may be only 30 to 60% of optimum levels in solution at lower nickel concentrations. This will affect the deposition rate, deposit coverage, brightness and solution stability. Because of how replenishment components are formulated, making large nickel replenisher activity additions of 20% or higher at one time will cause stabilizer and brightener concentrations to be at 120 to 160% of optimum levels. If large additions are made, the solution will be out of balance, and the bath's performance and deposit quality will be poor. Higher than optimum stabilizer and brightener levels cause voids, pits or skip plating, especially around sharp outer corners and edges. The metallic materials used as stabilizers are catalytic poisons at higher concentrations. In extreme cases, they will cause the plating reaction to stop.
Stabilizers are adsorbed on surfaces to be plated in tiny, scattered sites. The adsorption, in small amounts, helps accelerate deposition. Because the stabilizers are diffusion controlled, the more solution that passes over a surface, the more stabilizer can be adsorbed at that location. The edges of the work are the first locations where stabilizers are introduced due to agitation patterns. Under conditions of high agitation, low loading and high or normal stabilizer concentrations, too much stabilizer is adsorbed on the surface of the work producing too many active sites. This poisons deposition in those areas.
Nickel controls. As the nickel concentration decreases, the rate of nickel deposition decreases. A decreasing nickel concentration affects the coverage, brightness and uniformity of the deposit through a reduction of the plating rate or initiation of plate. In order to maintain these properties at an optimum level, small frequent additions should be made. In addition to stabilizer effects that can result, large chemistry replenishments tend to shock the solution, possibly resulting in salt precipitation (whiteout), deposit porosity and roughness, especially in aged baths.
Nickel concentration is determined by simple chemical analysis. A small sample of the solution is cooled to room temperature and analyzed with a standardized EDTA solution. The results can be expressed in percentage nickel activity. The optimum level for most EN systems is six g/liter nickel metal, which is equal to 100% activity. In most proprietary systems, the nickel analysis is also used to measure (or gage) the reducing agent, stabilizer, complexor and brightener additive concentrations. The consumption of these materials is predictable, but they are not always consumed at the same rate as nickel. In some situations, separate analyses and adjustments may be required to bring the solution into balance.
Maintaining the tank's solution level, in addition to sampling the solution before analysis, is critical. Also, the effect the tank's solution has on quality is often underestimated. When the solution is heated, water evaporates. Evaporation accelerates if the solution remains heated and at the operating temperature without working or replenishing the solution. Improper maintenance of the solution level through water additions allows all the chemical constituents to appear more concentrated. For example, a six g/liter nickel level may be 6.8 g/liter or higher as water evaporates. The concentration of all other chemicals will also be greater than the optimum level as water evaporates.
It is important to constantly maintain the proper solution volume, especially before plating or taking samples for chemical analysis. An overflow weir in the plating tank helps maintain a constant level that will minimize control problems.
Hypophosphite. The reducing agent is consumed both productively and non-productively during plating. The reducing agent influences plating efficiency. During plating, the sodium hypophosphite reducing agent, "the chemical rectifier," is consumed in a given ratio to the nickel metal during plating. The hypophosphite to metal ratio varies with proprietary systems, and specific supplier recommendations should be followed regarding the control. To maintain the proper metal to reducer ratio, the solution should be periodically analyzed and adjusted with hypophosphite. The reducing agent is also consumed non-productively through hydrolysis, which occurs when the solution is kept at the operating temperature and not used to plate. To optimize the hypophosphite efficiency, it is important to heat up and cool down the solution quickly while maintaining a constant process load in the tank when the solution is heated.
A reaction by-product formed during plating is orthophosphite, a salt that builds up in the solution. The build-up increases the density of the solution, which causes a reduction in the solubility of other components and reduces the plating rate. As the orthophosphite level increases, the deposit smoothness, brightness, plating rate and adhesion can be affected. Often, deposit roughness, pitting or porosity occur.
The steady increase of orthophosphite content is easily monitored by measuring the specific gravity of the solution at a constant temperature, usually room temperature. The orthophosphite content can be used to track the effective bath life and predict when, under normal operating conditions, the solution should be exchanged for a fresh solution. This effectively measures solution dragout or other solution losses on a production scale. Under laboratory conditions, where solution dragout can be controlled, the economical point at which to dump a solution is usually between six and eight metal turnovers. This is the point at which the least soluble salts begin to precipitate from solution. The solution becomes turbid or milky, producing poor quality deposits. There is little economical advantage to stretching the life of an old solution that becomes more difficult to control and maintain.
Contamination. The concentration of trace metals and other contaminants within the plating solution will affect deposit quality and appearance. Some metals may also tend to act as stabilizers and/or catalytic poisons that inhibit plating. Elements that act as stabilizers include sulfur, cadmium, bismuth, antimony, mercury, lead, zinc and iron. Sources of organic contamination include masking agents, oils, plasticizers from hoses and liners, airborne organic materials and silicates. Nitric acid contamination from stripping the EN tank slows the plating rate, increases deposit porosity and may cause black-streaked deposits. Excessive nitric contamination also results in poor adhesion of EN to the substrate. Poor adhesion occurs because the nickel deposit initiation is interrupted or slowed due to passivation or smut formation on the substrate's surface.
Nitrate test papers are a good tool to verify nitric contamination prior to making up a new EN solution. Neutralizing a tank with ammonium hydroxide or caustic soda does not ensure that all nitrate residues are eliminated from solution. When nitric acid is introduced to the EN solution as a contaminant, it exists as the anions of nitrate and nitrite. As little as one to two mg/liter of nitrite, which is stronger acting than nitrate, become a very strong stabilizer or poison in the EN solution. One to two grams of sulfamic acid added to a nitric contaminated EN solution react with nitrite to produce nitrogen gas that is evolved over time. To prevent the effects of nitric acid contamination, it is good practice to use a final rinse of sulfamic acid in the EN tank prior to making up a new solution. Use nitrate test papers to confirm the cleanliness of rinse tank and filtration systems before mixing a new EN solution. There are proprietary mixes that do not contain nitric acid for stripping EN tanks. In addition to safety issues, these types of proprietary mixes offer other advantages over nitric acid.
Operating pH. The solution's operating pH is an important parameter because it affects the plating rate and the amount of phosphorus co-deposited. Higher pH values favor lower phosphorus contents in the deposit while increasing the plating rate. Higher pH values, within the range for the particular system used, can lead to precipitation of metal hydroxides or orthophosphites. This precipitation normally causes porosity, which affects corrosion resistance.
Lower operational pH levels increase the phosphorus content in the deposit while decreasing the plating deposition rate. Slower plating rates due to operation at reduced pH produce less porous, more corrosion resistant deposits. Unlike electrolytic nickel plating, it is normal for the EN solution pH to decrease as plating continues. Many proprietary systems are self-regulating with respect to pH. Large fluctuations in operating pH are usually an indication of some process problem. Insufficient rinsing of work, which results in contaminant dragin, or inadequate replenishment of the maintenance components should be investigated if there is a pH control problem.
The pH is easily checked electromet-rically or with pH papers, providing the pH increments can be read to at least 0.2 pH units. In laboratory studies, the paper value has been shown to vary depending on the brand or type as well as other variables.3 As the solution's salt content increases, the colors of the pH indicators tend to vary. The amount of time the paper is read after immersion in the EN solution must be standardized to obtain consistent results since color continues to change over time. It is also advisable to calibrate the values obtained by paper with a pH meter for more consistent results. When using the electrometric pH meter you must calibrate the meter with two buffers that cover the expected range. For the best results, the electrometric pH should be measured on a cooled sample. Most pH probes do not perform well or have a limited life when used at EN operating temperatures of 180F or higher. The use of a fast-flow reference electrode works best for older, high-density EN solutions.
Operating temperature. The temperature of an EN plating solution is one of the most important factors affecting deposition rate. The deposition rate increases as the operating temperature increases, especially as temperatures reach 200F. It is important to have the proper heating system to avoid localized overheating, which can result in bath decomposition or deposit roughness. There is a wide range of plating rates versus operating temperatures for the various proprietary systems. For example, many of the four to six pct phosphorus systems plate at 160F while the 10 to 12% phosphorus systems need a minimum operating temperature of 180F. The deposition rate also has an influence on the deposit smoothness, coverage and adhesion. Too high a deposition rate can result in a rough or pitted nickel-plated surfaces. Too low a deposition rate slows the initiation of the EN on the substrate surface, which minimizes the coverage and resulting adhesion. If the nickel initiation is not quick enough, substrates can be attacked by the EN solution, affecting subsequent adhesion.
Bath loading and agitation. For EN solutions, the plating tank size should be no larger than necessary to meet production needs. If additional production is needed later, additional tanks should be used. It is not recommended to anticipate future increases by using a tank larger than current production requires. Bath loading and agitation have an effect on the deposit quality due to interactions with the chemistry. Figures 2 and 3 show the interaction between stabilizer concentrations with respect to loading and agitation.
Loading is referred to as the ratio of total exposed surface area being plated to the volume of solution in the tank. Different deposit characteristics can be obtained depending upon the solution loading. Optimum loading will depend on the type of bath or proprietary formulation. However, 0.3 to 0.8 sq ft/gal is a recommended average loading. As shown in Figure 2, if the work load to solution volume ratio is small (less than 0.2 sq ft/gal), the potential for stabilizer adsorption is greater, especially on edges and sharp geometries of the work. The poor coverage can result in edge pullback, step plating, inconsistency in plate thickness and increased porosity with a decrease in phosphorus content of the deposit. Higher bath loading only presents a problem if replenishment schedules are not kept up or if the solution chemistry fluctuates excessively due to high-volume additions. In many cases, high-bath-loading ratios cause the plating solution volume to grow due to required replenishments. This growth usually requires some solution be decanted to allow for the fresh replenishment. If solution removal is required, additions of the complexor make-up component are required to account for the dragout.
Agitation is important to maintain uniform heating and mixing of the solution. However, higher agitation patterns tend to exaggerate stabilizer edge effects as shown in Figure 3.4 A bath that has optimum loading and normal stabilizer concentration can produce poor quality deposits if the solution agitation is too high. As agitation increases, EN solutions can tolerate lower levels of stabilizer. When stabilizer concentrations are high, or if there is extremely low loading, there is more available stabilizer per area to be plated. Coupled with high agitation, this condition will frequently cause poor quality deposits.
Equipment. The equipment used to plate EN deposits can also affect deposit consistency and quality. Usually, the equipment is the difference between producing good or bad quality deposits. Steady maintenance of the tanks, heating, filtration equipment and racks is critical to success. Equipment materials should be resistant to the EN plating solution and the elevated temperatures used in plating. More importantly, they must be resistant to the solutions used to strip extraneous nickel from the tanks.
The air used to provide solution agitation must be clean and supplied from a non-oil-containing air compressor or blower. Oil cannot be efficiently filtered from compressed air since a portion of the oil remains in vapor form, which passes through the filters. There are filters made that will remove oil, but the expense is not justified compared to low-pressure, oil-free blowers.
Disposable, flexible PVC liners can be used in EN applications. However, caution should be taken when choosing flexible liners. PVC tank liners may cause pitting, non-uniform nickel coverage or other poor quality deposit characteristics.5
The heating systems should be designed to avoid localized overheating of the plating solution, which may lead to decomposition of the chemistry resulting in pitted, rough or porous deposits. External steam heat exchangers are the choice of many shops because they heat up the solution quickly. They also require little space outside the tank, which allows for complete use of the tank area for production. It is important to provide adequate velocity of the EN solution through the heat exchanger to avoid localized overheating. It is also recommended to place the filtration system in the same line as the heat exchanger to filter out particles that may form in the chamber of the heat exchanger.
Continuous filtration of EN solutions is required if optimum results are to be obtained. Particulate solution impurities, such as those introduced from sandblasted substrates or dust, can cause roughness in the final deposit. Most systems use five-micron retention filter media. If plating deposits greater than 25 micrometers, or other critical applications, the solution turnover rate should be at least 10 times/hr through a one micron or less filter retention size.
Adhesion. The importance of pre-plating operations cannot be over-emphasized in regards to EN adhesion to any substrate. Having the best EN system will matter if there is poor surface preparation. Some of the costs include stripping the defective nickel, possibly damaging or etching the substrate. Additional polishing, labor and chemical costs may be required. The surface preparation is said to be a factor in 95% or more of the problems with EN deposit adhesion, smoothness and brightness. The final EN deposit quality is only as good as the quality of the base substrate metal since EN's ability to level imperfections in the base material is poor. Smoother substrates produce smoother EN deposits.
Any defect in the substrate will likely be more visible after the part is plated bright. Few, if any EN systems will cover up base metal problems such as porosity, slivers from machining or polishing patterns. The manner in which the part is stamped, cast or drilled has an impact on the final plated product. Oils or compounds can be embedded into the surface of the part causing dull or non-adherent coatings. Good surface preparation is necessary.
Generally, a newer solution favors good adhesion since there are fewer reaction by-products of sulfate and orthophosphite. The sulfate concentration determines the degree of attack on various substrates at plating initiation. Increasing the orthophosphite concentration slows the plating rate and speed of initiation. These constituents can have an effect on adhesion.
Poor brightness and coverage. The basic EN cycle can be thought of as a series of steps intended to remove contaminants or films from the surfaces of the work. The cycle normally consists of a soak cleaner, electrocleaner and acid activation step. Rinsing is critical in the surface preparation cycle and is often overlooked as a source of problems on the line. Residues from cleaners, acids or other pretreatment steps can create passive areas on the part that will not initiate nickel deposition resulting in poor coverage, pitting or lack of deposit brightness. Sharing common rinses between the soak, electrocleaner and acids will cause deposit or solution performance problems. Detergents (surfactants) designed to clean soils in alkaline cleaner solutions may precipitate or form an oily layer if mixed with an acid rinse solution. Rinsewater temperatures of 60F or less require longer immersion times to assure effective rinsing. Very cold rinses will "setup" certain pretreatment residues on the surfaces of parts rather than removing them. Rinse stages are best if kept at 70F or higher. Air-agitated, counterflow rinses minimize contamination dragin to the EN solution. Process lines that incorporate a pre-dip of carbonate, bicarbonate or ammonium hydroxide prior to entering the EN solution have fewer problems with nickel deposit initiation, coverage, brightness and adhesion.
Performance testing EN solutions. Beaker testing of EN solutions can identify and correct problems. These techniques can be used to determine the plating rate, visualize stabilizer problems or verify solution life. It is important to standardize these techniques to provide reproducible results. However, the parameters can be changed to accomplish specific goals toward identifying a problem. A one liter beaker works well for this application because the narrow neck reduces the evaporation. These beakers are specially suited for bath life studies. A magnetic stirrer hot plate is used to agitate the solution. The test panels chosen can be steel or brass Hull cell panels (267 ml size). Steel panels are normally used to calculate rate and verify stabilizer effects. The brass panels are good to use for identifying pitting, especially for thick deposits. The preparation of these test panels is critical, and the results can be misleading if the panels are not properly cleaned and activated. Nickel wire is used to suspend the panels in the beaker.
With this technique, beaker loading can be varied by cutting the size of the Hull cell panel. Panels can also be bent 90 degrees to verify the effects of upper and lower shelf areas of the panel. Once the loading (area per unit volume in sq ft/gal or sq cm/liter) is chosen, the amount of agitation depends on the purpose for performing the beaker test. Some form of agitation should always be used. Laboratory work has shown agitation effects on the deposit rate and other important deposit characteristics.6 For stabilizer effects, choosing a low load of 0.25 sq ft/gal with moderate to high agitation is a good beginning point. For plating rate determinations, 0.5 sq ft/gal with moderate agitation is a good starting point for beaker testing. Panels can be weighed before and after plating to determine the rate of deposition by the deposit weight-gain method. There are different factors used in the calculation depending upon how much phosphorus is in the EN deposit. This method has been studied and correlates well with actual EN thickness by cross-sectional analysis.7 It is important to maintain the operating temperature within two degrees to ensure consistency and reproducibility of the testing. There can be many variations with these tests. However, they can be very useful to determine the quality of the EN deposit.
Given proper control, EN solutions can provide consistent, high-quality nickel-phosphorus deposits. Controlling the chemistry and operating variables of tank loading, agitation, pH and solution temperature are critical for producing high-quality deposits. The type and condition of the process equipment used, other types of mechanical influences and the surface preparation process are also shown to affect the performance of the EN solution. Paying attention to the details allows a high-quality EN deposit to be produced.
REFERENCES
- Riedel, W., "Electroless Nickel Plating" ASM International-Finishing Publications LTD., 1991.
- Mallory, G.O., "Electroless Plating Fundamentals and Applications," Edited by G. Mallory & J. Hajdu, AESF, Chap. 2, (1990).
- Justice, B., Products Finishing, 35, 7, pp. 80-83, (1982).
- Ibid 2, Chap. 1, (1990).
- Steinecker, C.P., Metal Finishing, 45, (May 1992).
- Kalantary, M.R., Holbrook, K.A., and Wells, P.B., "Effects of Agitation On Conventional Electroless Nickel Plating," Plating & Surface Finishing, pp. 5562, (August 1992).
- Allied Kelite EN Training Manual, Internal Training Document, 1981.
RELATED CONTENT
-
Cleaning, Pretreatment to Meet Medical Specs ISO 13485 or FDA 21 CFR820
Maximilian Kessler from SurTec explains new practices for industrial parts cleaning, metal pretreatment and decorative electroplating in the medical device industry.
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Zinc Electroplating
Choosing the best process for your operation.