Cyanide Destruction: A New Look at an Age-Old Problem
Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
#research #pollutioncontrol #surfin
Editor’s Note: The following is a synopsis of a presentation given at NASF SUR/FIN 2018, in Cleveland, Ohio on June 4, 2018 in Session 3, Waste Management: Perspectives Connecting Finishers. A pdf of this brief can be accessed and printed HERE; the complete Powerpoint presentation is available by clicking HERE.
ABSTRACT: Cyanide in mining and industrial wastewaters has been around from the beginning, including electroplating processes. This presentation reviews a number of current processes, and in particular, offers new technologies for improvement in cyanide destruction by the most common process, using sodium hypochlorite.
Featured Content
Introduction
Cyanide chemistries in mining, as well as industrial process wastewaters, including plating operations, have been used since before the start of the industrial revolution. Plating baths derived from cyanide chemistries have been an efficient and economical means of depositing certain metals in commerce. However, issues of toxicity and occupational health have rendered them undesirable, and the search for substitutes has proceeded apace in recent years. However, there remain applications for which cyanide chemistries provide the only means of meeting the property and performance specifications required. Consequently, the effective destruction of cyanide wastewaters is still an important issue to the surface finishing industry
Review of cyanide destruction processes
There are a number of cyanide destruction process in use today. These include:
- Alkaline chlorination
- Biological treatment
- Caro’s acid
- Hydrogen peroxide
- Sulfur dioxide and air
Alkaline chlorination
The most common form of cyanide destruction for plating processes involves alkaline chlorination. Traditional treatment involves a high pH of 10.5, and a high oxidation-reduction potential (ORP) of +600 mV. It is a chemically heavy process, using approximately 23 gallons of 12.5% sodium hypochlorite solution to destroy one ounce of cyanide. Sodium hypochlorite usage at this rate is quite high.
The first step in the process produces cyanogen chloride:
(1)
The cyanogen chloride then hydrolyzes to form cyanate:
(2)
The second step involves the hydrolysis of cyanate ions to form ammonia and carbonates:
(3)
Our new proprietary technology addresses a number of problems associated with pH and hypochlorite usage.*** The operating pH is considered reduced, at 9.5. Most importantly, the advanced technology reduces the equivalent amount of sodium hypochlorite required by 94%, at 1/16 the level of the traditional process.
Biological treatment
Biologic treatment through the use of bacteria has been used as an effective means of cyanide destruction. Aerobic bacteria, including pseudomonas, alcaligenes, achromobacteria and others, are used to form cyanates through oxidation:
(4)
The cyanate ion is biologically converted to ammonia and bicarbonate:
(5)
Caro’s acid
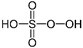
Skeletal formula of peroxymonosulfuric acid
Caro’s acid, or peroxymonosulfuric acid (H2SO5), was introduced in the early 1990s as a cyanide destruction agent. It is produced by an exothermic reaction involving hydrogen peroxide and sulfuric acid. It is very unstable and needs to be used immediately, as the cyanide destruction is a rapid one, reaching completion in just a few minutes:
(6)
The oxidation of cyanide to cyanate involves a one-to-one molar ratio in this process. The highly reactive chemistry is a major disadvantage in using this technology on the scale of plating operations.
Hydrogen peroxide
The hydrogen peroxide process is widely used at steel hardening facilities, primarily as a case-hardening process for low carbon steels. Typically, the process involves a molten salt bath of sodium cyanide operating in the range of 1600-1750°F. Again, environmental issues have led to considerably less usage of this technology.
Cyanide destruction by hydrogen peroxide requires a catalyst such as copper to increase the reaction rate. The metals bound by the cyanide must be precipitated as a metal hydroxide. Once again cyanide is converted to cyanate:
(7)
For the bound metals,
(Solid) (8)
Sulfur dioxide and air
There are two patented versions of this process, held by Inco and Noranda, related to nickel processing. In particular, the mining operations use the process to treat tailing slurries. The process is effective for treatment of free and weak-acid dissociable (WAD) cyanides, and a catalyst is required to achieve a practical reaction rate. The reactant from air is oxygen. Again, cyanide is converted to cyanate:
(9)
For the bound metals,
(10)
The optimal process pH is in the range of 8-9. The sulfur dioxide can be in the form of liquid sodium dioxide or derived from sodium sulfate or sodium metabisulfite.
Summary
The use of cyanide chemistry in industrial processes, while posing environmental and health problems, is still in use in many mining and industrial arenas, including plating operations, owing to the fact that specific alternatives are few or non-existent. This paper reviews technologies used in the mining and surface finishing industries.
Cyanide destruction is the primarily cyanide destruction technology in electroplating, and generally involves the use of sodium hypochlorite chemistry. A process is discussed here which allows operation with reduced alkalinity and a significant reduction in sodium hypochlorite usage, reducing a number of safety and chemical consumption concerns.
About the author

Robin Deal is a Wastewater Field Service Technician with Hubbard-Hall, Inc., in Inman, South Carolina. She holds a Physical/Chemical Waste Water Operator’s license for North Carolina and is currently working toward a Bachelor of Science degree from the North Carolina Agricultural and Technical State University (2017-2020).
*Compiled by Dr. James H. Lindsay, Technical Editor - NASF
** Corresponding author
Robin Deal
Technical Field Service
Hubbard-Hall
1101 Compton Bridge Road
Inman, SC 29349
Phone: (864)-472-2258
E-mail: rdeal@hubbardhall.com
***AquapureTM Wastewater Treatment Processes, Hubbard-Hall, Inc., 1101 Compton Bridge Road, Inman, SC 29349.
RELATED CONTENT
-
History of Chromium Plating
A review article originally printed as Plating & Surface Finishing, 71 (6), 84-91 (1984) on the occasion of the 75th anniversary of the founding of the American Electroplaters Society.
-
Electroplated Tin-Nickel Coatings as a Replacement for Nickel to Eliminate Nickel Dermatitis
This paper is a peer-reviewed and edited version of a paper delivered at NASF SUR/FIN 2013 in Rosemont, Ill., on June 12, 2013.
-
Chromium-free Etching and Palladium-free Plating of Plastics
Plastics are replacing metals in the manufacture of many parts, and quite often there is a need for metallic coatings on the plastics and other non-conductors. This paper will describe new processes of preparing ABS plastic substrates for subsequent metallization.



















