An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
#basics
By definition, electroless plating is metal deposition by a controlled chemical reaction. In contrast to an electroplating solution, electroless nickel (EN) solutions require no external source of current to plate. EN baths utilize a chemical reducing agent built into the bath. The process provides a continuous build-up of deposit, since the metal being plated is itself a catalyst for the plating reaction. This is why EN is also known as autocatalytic nickel plating.
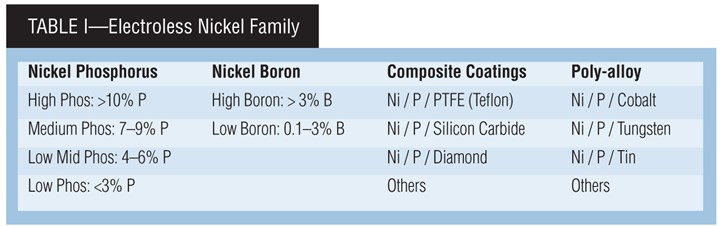
Electroless Nickel Family
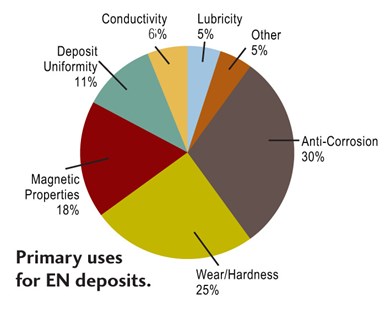
There is a large variety of EN coatings, typically defined by their alloy. They all share several properties, for example a high degree of deposit uniformity regardless of part geometry. Applications for EN can be found in virtually every industry due to a unique combination of deposit properties, including: excellent corrosion protection; superior wear resistance; uniform deposit regardless of part geometry; hard deposits as plated, with heat treatment available to increase hardness; plating on catalyzed non-conductors, such as plastic; solderable deposits; ability to change the magnetic properties of a part and provide a diffusion barrier; and ability to provide salvage of worn or mis-machined parts.
Featured Content
Nickel Phosphorus. The majority of EN plating is done using nickel phosphorus (Ni-P) systems. These deposits give a low coefficient of friction and are anti-galling. They have superior as-plated hardness and can be further hardened by post-plating heat treatment processes. These deposits have excellent corrosion performance in many types of environments.
Nickel boron alloys are widely used in electronics and aerospace applications. The deposits provide high electrical conductivity, low contact resistance, excellent as-plated hardness, a high melting range, outstanding wear resistance, and are easily soldered or brazed.
Composite EN coatings can contain co-deposited soft particles, such as polytetrafluoroethane (PTFE), or hard particles, for example silicon carbide. EN/PTFE deposits provide a coating with a very low coefficient of friction. Co-deposited hard particles provide improved wear resistance.
Ternary alloys. Also called poly-alloys, these deposits contain more than two elements. An example is nickel phosphorus tungsten, which provides a very hard coating.
Bath Chemistry
Nickel deposition by hypophosphite is usually represented by the following reactions:
- NiSO4 + H2O → Ni2+ + SO42 - + H2O
- NaH2PO2 + H2O → Na+ + H2PO2- + H2O
- Ni2+ + H2PO2- + H2O → Ni + H2PO3- + 2H+
- H2PO2- + H2O (CATALYST)→ H2PO3- + H2
A typical EN plating bath consists of a source of soluble nickel ions, a reducing agent, complexors, neutralizers/buffers, stabilizers, and (in some cases) brighteners.
Nickel source. In EN plating, the metal source is a soluble nickel salt. The choice of what salt to use is based on solubility, purity, compatibility and price. Nickel sulfate is the most widely used nickel salt, but processes using nickel chloride, nickel sulfamate, nickel acetate and nickel hypophosphite are commercially available.
The reducing agent replaces the rectifier used in electroplating. Widely used reducing agents are sodium hypophosphite, sodium borohydride and dimethylamine borane.
Complexing agents keep the nickel in a stable complex until it's needed for plating. The choice of complexors determines the deposit alloy and thus its properties. For example, in hypophosphite-reduced systems stronger complexors are used in high-phosphorus systems, while weaker nickel complexors are used in low-P deposits.
Neutralizers/buffers. While plating, an EN bath will generate hydrogen both as a gas and as ionic hydrogen. This will lower the pH of the solution. Buffers can be used to minimize pH swings in the plating bath, but excess acidity must be neutralized to maintain the correct pH. Typical neutralizers are ammonium hydroxide, carbonates or sodium or potassium hydroxide.
In North America, ammonium hydroxide is currently the most commonly used neutralizer. However, due to its objectionable smell and its negative impact on waste treatment, more EN applicators are switching to alternatives. Methods and additives exist which allow EN platers to utilize sodium hydroxide as a viable alternative for pH adjustment.
Stabilizers control the plating reaction. Without these catalytic poisons, the reaction may be uncontrolled. Stabilizers are divided into two basic categories: metallic and organic. Historically, lead compounds have been used as metallic stabilizers.
Brighteners. Many EN systems use a brightener to enhance deposit appearance. Brighteners can be either metallic or organic compounds. Cadmium compounds are commonly used.
Environmental Drivers
Recently there has been a major shift in EN technology, due primarily to European environmental legislation. Among the most influential legislation are the RoHS, WEEE, and ELV directives. The Restriction of Hazardous Substances (RoHS) and Waste Electrical and Electronic Equipment (WEEE) directives are intended to promote the reuse, recycling and recovery of electrical components. The European End of Life Vehicle Directive (ELV) is aimed at waste minimization through recycling, thereby eliminating hazardous waste from landfills.
The RoHS directive limits the maximum concentration values for lead, mercury, hexavalent chromium, polybrominated biphenyl or polybrominated diphenyl ether to 0.1 wt.% (1,000 ppm), and cadmium to 0.01 wt.% in homogeneous materials. The June 2002 ELV Annex II stated “...lead, cadmium, hexavalent chromium and mercury must not be intentionally introduced or deliberately utilized in the formulation of a material or component…” In September 2005, the verbiage of Annex II was revised to omit the phrase “intentionally introduced,” and now reads “A maximum concentration value up to 0.1% by weight and per homogeneous material, for lead, hexavalent chromium and mercury and up to 0.01% by weight per homogeneous material for cadmium shall be tolerated.” In both cases, a plated layer is considered a homogeneous material.
Most existing lead-stabilized, hypophosphite-reduced EN systems should provide a deposit containing less than 0.1 wt.% lead. Deposits from conventional cadmium-brightened systems would almost certainly contain in excess of 0.01 wt.% of cadmium. In all probability existing lead-stabilized, hypophosphite-reduced EN systems which do not contain cadmium are RoHS- and ELV-compliant, while cadmium brightened processes would likely be noncompliant. While many existing lead-stabilized baths that contain no cadmium are probably compliant, it is possible that future legislation and/or specifications will mandate the elimination of lead and cadmium from EN deposits. Virtually all major EN suppliers offer RoHS- and ELV-compliant chemistries with no lead or cadmium.
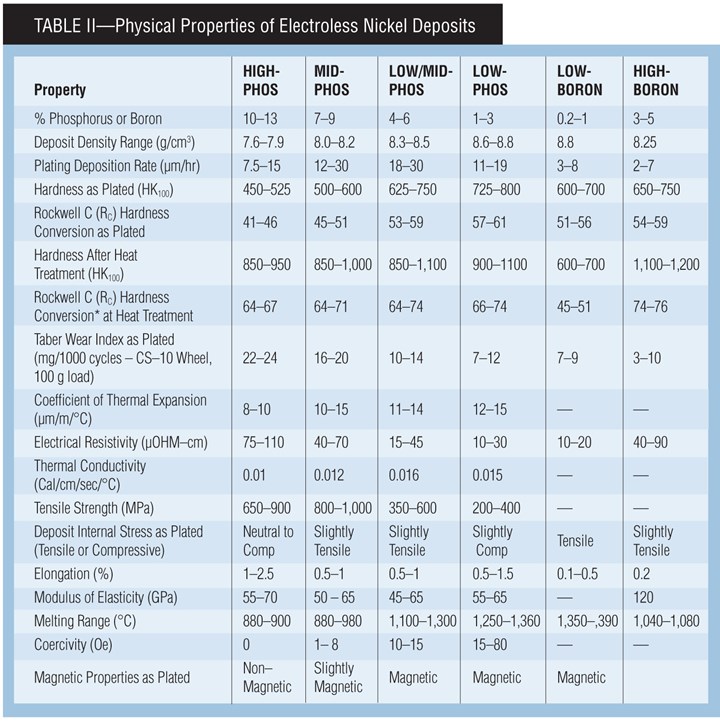
Deposit Properties
Since electroless nickel encompasses a broad family of coatings, the deposit characteristics will vary according to process. Since the primary factor determining coating performance is the deposit alloy, deposits from RoHS/ELV-compliant systems and conventional systems share certain properties, such as smoothness, high hardness and a high degree of uniformity. These deposit properties give several key attributes, including excellent corrosion protection, superior wear resistance, and consistent deposit thickness regardless of part geometry.
Corrosion protection refers to how well the coating protects the plated article. Corrosion occurs when metals react electrochemically, resulting in an electron transfer that causes corrosion.
There are two basic methods to describe the corrosion protection mechanism provided to a substrate. An anodic or sacrificial coating (such as zinc) protects a part by preferentially dissolving in lieu of the substrate. A barrier or cathodic coating protects a substrate by forming a protective layer between the part and the environment. EN normally protects the substrate from corrosion by acting as a barrier coating.
Electroless nickel offers excellent corrosion protection provided there is total encapsulation of the substrate. As with any barrier coating, the protective value EN offers depends on initial surface quality. Smooth, pore-free surfaces tend to perform best, while rougher, more porous substrates tend to give relatively poor results.
Assigning absolute figures for the corrosion protection of EN deposits can be misleading, since the majority of corrosion failures can be traced back to substrate porosity or improper pretreatment resulting in deposit porosity. In most applications, high-phosphorus deposits generally provide the highest corrosion protection. Electroless nickel deposits are especially well suited in applications where a component will be exposed to corrosion as well as wear.
Post-plating operations can impact the corrosion performance of the coating. High temperature heat treatments for hardness (>400°C) can crack the coating, compromising the barrier coating and lowering its effectiveness. Conversely, adding a supplemental coating, such as oils, waxes, and lacquers can help. On certain substrates, such as aluminum, corrosion performance can be enhanced by using a post plate passivation, which would passivate the substrate through any potential surface porosity.
Corrosion resistance can be described as how well the deposit resists attack. Electroless nickel coatings offer good corrosion resistance in many harsh environments. In most environments, high-phosphorus deposits provide the best resistance to chemical attack, but low-phosphorus deposits often show advantages in alkaline environments.
Wear Resistance. Electroless nickel coatings provide excellent resistance to most types of wear, both in the as-plated condition as well as after heat treatment. There are many different types of wear:
-
Fretting—caused by vibration between two contacting surfaces
-
Erosion—caused by impinging particles, liquid, or gas
-
Surface fatigue—caused by fracture or degradation in the surface
-
Abrasive wear—from particles or protub-erances rubbing the surface
-
Adhesive wear—caused by contact bonding between two surfaces.
Microhardness. Hardness values for EN should be measured using the Knoop or Vickers microhardness methods, because surface hardness readings such as Rockwell are not accurate. Typically the EN deposit is too thin for reliable surface testing, resulting in the reading being influenced by the substrate. in the as-plated condition, lower-phosphorus deposits are typically harder than higher-phosphorus deposits as plated. Deposits can be heat treated to improve hardness, both by creating a more crystalline nickel deposit and by forming nickel phosphide in the deposit.
Phosphorus Content vs. Deposit Properties. The properties of Ni-P EN deposits vary based on the percentage of phosphorus. Below are some general guidelines of the effects of phosphorus content in the deposit on physical properties:
-
Hardness—deposit gets harder as phosphorus content decreases
-
Wear—deposit is generally more wear-resistant as phosphorus decreases
-
Electrical—conductivity increases as phosphorus content decreases
-
Solderability—deposit becomes more solderable as phosphorus decreases
-
Melting Range—melting range increases as phosphorus content decreases
-
Corrosion Resistance—deposit is generally less corrosion-resistant as phosphorus decreases
-
Corrosion Protection—typically, corrosion protection decreases slightly as phosphorus content decreases
-
Elongation—ductility is highest at less than 2% P and greater than 10% P
-
Magnetic tendency—deposit becomes more magnetic as phosphorus content decreases.
Controlling EN Baths
Since electroless nickel plating relies on a chemical reduction reaction, control of the process is critical in obtaining optimum results. A typical EN bath is more sensitive to operating conditions than an electroplating bath, and care must be taken to control the process within relatively tight parameters to achieve the best performance.
Bath age is typically tracked by metal turnovers (MTOs). In a 1-L plating bath operating at 6 g/L of nickel metal, one MTO occurs for each 6 g of nickel added back to the system. As the plating reaction proceeds, by-products will form and will eventually degrade the performance of the plating solution and the deposit. In a sodium hypophosphite-reduced bath using nickel sulfate, the by-products include sulfate, sodium and orthophosphite. About 45–60 g/L of reaction by-products are formed every MTO. Depending on criteria, EN baths will normally last from 4–10 MTOs before their performance degrades beyond acceptable limits and the bath must be discarded.
There are methods available to extend useful bath life. One method entails reducing the amount of by-products generated by utilizing an alternate nickel source, such as nickel hypophosphite or nickel acetate, either of which would eliminate sulfate and dramatically reduce the amount of sodium generated. These systems work very well, but the downside is the higher cost of the nickel salt. There are also purification methods available, such as “bleed and feed,” precipitation, and electrodialysis.
Several years ago, a new method to extend bath life was developed. A typical hypophosphite-reduced EN bath running at 6 g/L of nickel would contain about 120 g/L of dissolved solids at make-up, depending on the bath formula. Each MTO of operation adds around 45 to 60 g/L of dissolved solids. Reducing the amount of dissolved solids present in a new solution will result in a system able to hold more reaction by-products. Operating at lower metal concentrations has been used for years as one method of reducing the total dissolved solids in a new plating bath. In a plating bath designed to run at 3 g/L nickel, dissolved solids on make-up will be about 75 g/L—approximately 45 g/L less than a bath operating at 6 g/L. The reduction of dissolved solids on make-up allows the solution to hold more reaction by-products, resulting in a bath life extension of one-half to one MTO. There is also less drag-out, less nickel misting, and potentially lower waste treatment costs.
Bath age can be determined by maintaining accurate replenishment records, analyzing the orthophosphite concentration, or by measuring the specific gravity of the bath.
Bath concentration is typically controlled by analysis of the nickel, because titration for nickel is a relatively quick, easy test. Analytical frequency depends on bath loading and plating rate. If additions of greater than 10% activity are routinely being made, the frequency of analysis should be increased. The ideal operation is a steady-state condition, adding replenishment chemistry at the same rate it is consumed by plating. The better the control of the EN process, the better the process will perform.
Reducer concentration should be checked, generally once every MTO. Reducer should be consumed at a rate proportional to the nickel, but different operating variables, such as bath concentration, tank loading, agitation method and amount of idle time at operational temperature, can impact the amount of reducer consumed in the tank.
Operating temperature is the primary factor in determining plating rate. Low temperatures provide less energy to the chemical deposition reaction and result in lower plating rates. Very high temperatures can make the bath too active, possibly resulting in plate-out and general bath instability. Automatic temperature controllers, frequently calibrated, are strongly recommended.
Operating pH. Aside from bath formulation, operating pH is the most influential factor on deposit phosphorus content. Typically, higher pH ranges give lower phosphorus content in the deposit, while lower pH values result in higher-phosporus deposits. The pH of an EN plating bath should be checked every time a nickel titration is performed.
Bath volume. Maintaining the operating level of the plating bath is a critical and often overlooked control factor. Consider a plating tank 50 inches deep. At the 50-inch level, the solution is at 100% activity and the bath is chemically balanced.
A load is plated and consumes 10% of the bath chemistry. During plating, the solution level of the tank drops 5 inches, or 10%. When the evaporated bath is analyzed, it would show 6 g/L of nickel metal. However, the bath would not be in balance. Specifically, the stabilizers would be low, and the ratio of chelates in the bath would be higher than normal. The bath is now unbalanced, and the low stabilizers could lead to bath instability.
Ongoing Change
In conclusion, the future of EN plating is being shaped by environmental and health legislation, which is driving change. These changes can open the door for new innovations. The overall goal of any change should not be limited to ensuring environmental compliance, but should also address such issues as reduced operational cost and waste generation, creating a better workplace environment, and utilizing “greener” processes.
RELATED CONTENT
-
Electroless Nickel Conference 2023: Learn, Solve, Network
The Electroless Nickel Conference 2023 takes place Sept. 26-29th in Milwaukee, Wisconsin.
-
Electroplating for Medical Devices
Brittany McKinney of Pavco Inc. discusses the benefits of electroplating for medical devices.
-
Electroless Nickel On the Rise
ENC 2023 was held in Milwaukee, Wisconsin, offering a wealth of educational programming and networking opportunities for finishers looking to grow their electroless nickel business.