EN Process Performance
A review of experiments that show the effect of agitation, loading and stabilizer level...
Numerous studies have been conducted on the chemistry of electro- less nickel (EN) and its relationship to deposit performance.1,2 The core of this work concentrates on mechanical and physical properties, such as wear and corrosion resistance. There is, however, limited information on the combined effects of agitation, loading and stabilizer levels on the performance of EN processes and the resulting deposits.
An experimental matrix consisting of nearly 200 experiments was conducted on a typical high-phosphorus EN process. Type and intensity of agitation, workload to solution volume ratios and stabilizer levels all were varied. The synergistic effect was evaluated for critical process characteristics-the six P's: porosity; passivity; pitting; pullback; plating rate; and plate-out.
Featured Content
Marginal performing EN processes based loosely on the original "Kanigen" style chemistries have all but been replaced by a myriad of specialty EN systems that offer properties specialized for many of today's demanding applications. Proprietary systems today are, for the most part, easy to make up and operate. Concentrates are properly balanced, and variations in chemistry are minimal when operated properly. However, modern EN still has its technical flaws.
A pure, stabilizer- (poison) free EN solution is desired to produce a deposit with ideal properties such as low stress and porosity as well as high passivity and zero pitting. However, the EN process must offer performance benefits such as reduced plate-out, high plating rates and, in some cases, bright deposits. Most often a balance exists between the two, but not without problems.
Poor corrosion protection due to high porosity; inferior chemical resistance due to inadequate passivity; pitting of thick deposits; edge pullback with small workloads; slow plating rates; plate-out at some point, all of us have encountered one or more of these afflictions. A summary of each follows:
1. Deposit porosity and inherent corrosion protection
EN deposit porosity determines corrosion protection for a majority of applications, including the galvanic series. Sacrificial coatings such as zinc or cadmium are most often anodic to the substrate and preferentially corrode, protecting the cathodic substrate. With barrier coatings like EN, the opposite holds true. EN is most often the cathode(1) and corrosion of the anode (the substrate) occurs through the pores and voids of the EN deposit. It is rare when the corrosion resistance of the actual EN deposit comes into play.
|
||||||
During the past decade, much has been made of methods to improve corrosion protection of EN deposits by reducing porosity. This has been accomplished through improved pre-treatment,3 various heat treatments4 and in-depth chemistry modifications.5 An objective of this study is to provide methods to minimize porosity through modified solution operation techniques.
2. Deposit passivity
Passivity is defined as "A condition in which a metal, because of an impervious covering of oxide or other compound, has a potential much more positive than that of the metal in the active state."6 As it relates to EN, passivity has been a quick and somewhat reliable method to test relative purity of a particular deposit. It has been used in the past as a rough measure of phosphorus content,(2) although a direct correlation is highly suspect.
The blackening of the deposit has less to do with the nickel stripping than the formation of nickel sulfide and nickel oxide on the surface.7 Factors contributing to the accelerated attack of EN deposits by nitric acid include co-deposition of heavy metals such as cadmium and lead as well as the presence of co-deposited sulfur, typically found in low- and medium-phosphorus systems.8 These experiments used a sulfur-free, organic compound, stabilized, high-phosphorus process that contained a trace amount of heavy metal as a complementary stabilizer. It used the nitric acid test as a measure of deposit purity and as a gage for comparing corrosion performance of EN deposits plated under different conditions.
3. Pitting of thick deposit
Although surface condition and substrate pretreatment play a role in EN deposit pitting,9 it is one of the most notable defects to emanate from the plating solution. Pitting is common in low- and medium-phosphorus deposits and in thick deposits of all EN types. High-phosphorus processes are less susceptible to pitting. Lower stabilizer levels, both heavy metal and sulfur-organic type, result in less co-deposition of impurities and a more homogenous deposit. The high-phosphorus chemistry also plates slower. This is significant because hydrogen gas bubbles are believed to attach at the substrate solution interface, causing pitting. As the gas bubbles form on the plating surface, they inhibit deposition until the gas bubble releases into the surrounding solution. The pit grows larger in size as the deposition process continues.
|
TABLE II
|
||||||||||
|
MTO |
NI
(g/l) 6.0 6.0 5.9 6.1 5.7 5.8 |
Hypo
(g/l) 30.0 29.4 30.3 30.3 29.4 31.5 |
Ortho |
Specific |
Plating
rate (mil/hr) .55 .52 .48 .46 .42 .40 |
HM
stabilizer (ppm) .35 .37 .31 .38 .41 .44 |
Passivity
(min) 8 N/A N/A N/A N/A N/A |
Pores
4 7 5 15 12 22 |
Pits
11 8 15 5 5 8 |
Plate-out
light plate-out light plate-out light plate-out |
The hydrogen gas attachment process and subsequent formation of pit pre-cursors can be brought on by a number of factors. Substrate morphology and adsorption of microparticles are both considered suspects.10 High-speed EN processes are very susceptible to pitting because the plating continues to grow rapidly while the hydrogen gas bubble adheres to the trouble spot. Slower plating speeds, like those found in high-phosphorus processes, allow time for the hydrogen gas bubble to release before a significant amount of nickel can be deposited in the surrounding area. Wetting agents that lower surface tension and promote a quicker gas bubble release help to an extent; however, the pits remain, and the source is not eliminated. We monitored the effect of loading, agitation and stabilizer level on deposit pitting. Efforts were made to identify sources of pitting and methods to minimize them through operational techniques.
4. Edge pullback phenomena
Edge pullback is produced by high concentrations of stabilizers, brighteners or metallic poisons. While this is only postulated, it is believed that stabilizers act by preferentially adsorbing at the surface of colloids or near colloidal micelles, preventing random formation of catalytic particles and subsequent solution instability.11 These stabilizers and/or poisons also readily co-deposit.
|
HM Stabilizer |
Pores
0 13 20 25 |
Pits |
Passivity*
(min) 1/8 1/8 0.75/5 0.33/4 |
Plating rate
(mil/hr) .51 .54 .47 .47 |
Pullback |
Plate-out |
* minutes to black edges/minutes to blackening of entire panel
This co-deposition process can be accelerated by excessive agitation and/or low workload to solution volume ratios. Most stabilizer performance is governed by diffusion. The more solution that passes over a surface, the more stabilizer can be adsorbed at that site. Areas of high solution movement, such as edges and holes, are the first locations affected. Stabilizers also tend to adsorb on sharp points and threads and can lead to skip plating, high porosity or poor passivity. By monitoring mixed potential, Mallory found that increased solution agitation shifts the critical point of a stabilizer (the point at which it poisons the reaction) to a lower concentration.12 The effect of various solution loading, agitation and stabilizer levels on edge effect phenomena will be evaluated and noted.
5. Plating Rate
Plating rate is a critical process characteristic and often determines the commercial viability of a particular chemistry. Various studies have found that specific organic acids offer significant advantages in terms of stress and corrosion protection only to be eliminated from commercial consideration due to slow plating rates.13,14
It is widely accepted that plating rate is a function of the following factors: temperature; pH; solution age; type and amount of organic acid; sodium hypophosphite concentration (most chemistries); presence of rate exaltants; and stabilizer type and level. Some have also been affected by agitation15 and workload to solution volume.16
Our studies focused on the last three. Numerous studies have shown that both stabilizer type and level have a profound effect on plating rate.17,18 In most cases, the research found that Class II (oxy-anion), Class III (heavy metal) and Class IV (unsaturated organic acid) stabilizers all reduced the plating rate as their levels approached the critical value (the point where plating stopped). In our experiments, we monitored plating rate as a function of stabilizer level within a tight range, one that an EN plater would typically encounter between 80-120% solution activity.
Stallman19 found that deposition rate increased with an increase in flow velocity. He found that several low intensity stirrings produced better results than one and recommended pumping solution through numerous outlets. It is believed that higher agitation increases plating speed by intensifying rates of diffusion of reacting species at the plating interface. Fresh reactants (nickel, sodium hypophosphite) are brought in, and by-products (H2, etc.) are removed, both at faster rates.
|
HM Stabilizer |
Pores
36 20 13 36 |
Pits |
Passivity
(min) 10 sec/2 40 sec/3 1/8 2/8 |
Plating rate
(mil/hr) .54 .46 .54 .56 |
Pullback |
Plate-out |
Some studies found that certain types of agitation can actually decrease the plating rate.20,21 Feldstein, et al. found a sharp decrease in plating rate with excessive stirring agitation and associated it with suppression of the nucleation sites as plating initiated. He found that if agitation was introduced after an initial layer of nickel was deposited, it had little or no effect on plating speed. He also varied rotation speed from 0-1,200 rpm and found a significant increase up to 300 rpm, at which point the plating rate dropped until no plating occurred. In this segment of our matrix we evaluated plating rate as a function of stirring speed (580 rpm, 900 rpm), air and nitrogen agitation.
A correlation between workload to solution volume ratio and plating rate has been claimed.22 Riedel referenced an Allied-Kelite study that showed a clear connection between higher loading and slower plating rates.23 The flaw in this experiment is that replenishment was made every 60 min and at a loading of 0.25 sq ft/gal and 0.55 sq ft/gal respectively. It is possible the solutions were in a constant state of low pH and reactants.
Gutzeit and Kreig found faster plating rates were realized with higher workloads.24 Effect of solution loading in the range of 0.05 to 1.0 ft2/gal was evaluated while chemistry was maintained at or near optimum levels.
|
Agitation |
Pores
13 6 16 16 15 |
Pits |
Passivity
(min) 2/8 2/10 1/5 1/10 2/10 |
Plating rate
(mil/hr) .54 .52 .60 .61 .53 |
Pullback |
Plate-out |
6. Plate-out or solution stability
Equipment plate-out occurs for several reasons. Solution chemistry (stabilizer type/level and complexing agent), operating parameters (temperature, pH) and equipment (tank condition, agitation amount, filtration) are several of the key players.
We observed the effect stabilizer level, agitation and workload have on solution stability and the propensity to plate-out on the bottom of a glass beaker. All other variables that could play a role in instability (equipment, etc.) were kept fixed and were therefore discounted.
Experimental Procedure
The total experiments in the matrix was determined, and lab-made lots of make-up and replenishment chemistry were set aside to guarantee all solutions were made from the same raw materials. Critical stabilizers were not added to these stock solutions so that their levels could be adjusted during specific experiments. A 50% solution of ammonium hydroxide was also prepared and saved. All EN plating solutions were high-phosphorus type (10.5-12% b.w.) and processed through a 1-micron absolute filter prior to use. In each case, 800 mls of EN solution was prepared and heated to operating temperature. Unless otherwise noted, all EN solutions were aged to 0.5 metal turnovers before initiation of tests.
Panels used for passivity tests, plating rate and pitting analysis were all polished 1010 carbon steel 3 × 4-inch zinc coated (removed during cleaning cycle). When necessary, panels were cut to size for certain workload requirements. Standard 1 × 0.25-inch zinc-plated 1010 mild steel hex bolts were used for porosity measurement as well as dummy bolts to meet certain workload requirements.
Ferroxyl test. Solution was prepared, and all tests were conducted per ASTM B733. Hex bolts (1 × 1/4-inch) were plated to 0.4 mil of high-phosphorus EN and tested for porosity. Visual examination was performed after 5 min air dry.
Pitting test. Panels (1x 0.75-inch) were plated to 2.0 mils and grids were applied with permanent ink. The deposit was then evaluated under a microscope at 20x for appearance of round, "fish eye" type pits. This type of pit has a distinct oblong center and is typical of pits brought on by the EN process. Pits due to roughness and/or surface imperfections were not counted.
Passivity test. Panels (1x 0.75-inch) were plated to 0.2 mil, dried and immersed in concentrated reagent-grade 70F nitric acid. Two variables were monitored: 1) The time required for the edges to turn black was considered a qualitative measurement of the stabilizer adsorption process; and 2) The time for the entire panel to turn black was also documented.
Plate-out/instability. Solution stability was measured by two methods: 1) Monitoring for extraneous nickel consumption and/or evidence of plate-out on the bottom of the glass beaker; and 2) A palladium stability test that was run in all cases except for loading experiments. This test required the addition of a dilute solution of palladium chloride to a hot EN solution over a set time until the solution decomposed. The higher number of mls of palladium chloride required, the greater the solution stability.
Standard Cleaning Cycle
The following is the cleaning and activating cycle followed for both panels and bolts (rinses omitted):
1. Immersion in 50% hydrochloric acid at ambient temperature for 30 sec to remove zinc.
2. Anodic electroclean (12 oz/gal) at 180F for 2 min at 3v.
3. Immersion in 50% hydrochloric acid until uniform gassing is evident.
4. Repeat step 2
5. Repeat step 3
The double cleaning cycle minimizes variations in results due to poor cleaning. C.F. Beer25 reported that anodic electrocleaning in highly alkaline cleaners was superior in promoting reduced porosity than either soak cleaning or cathodic electrocleaning. Although claims have been made that chlorides from hydrochloric acid activation can "accelerate" corrosion,26 we have been unable to find any correlation between the type of activating acid used and the porosity of thin, high-phosphorus deposits.27 More important factors include complete soil and oxide removal as well as adequate rinsing.
Preparation of High Phosphorus Solutions
Preparation of 800 ml bath included the following steps:
1. 36 ml of high-phosphorus nickel component and 120 ml of high-phosphorus make-up component
2. Required addition of stabilizer
3. Adjust to volume with DI water
4. Filter through 1-micron absolute filter
5. Adjust pH to 4.8 with 50% b.v. ammonium hydroxide
6. Heat to 190F and begin plating 2-3x4-inch steel panels
7. Monitor solution concentration per standard EDTA titration and replenish as needed up to 0.5 metal turnovers.
8. Cool solution, analyze, replenish and adjust pH to 4.8
9. Plate 30 min rate panel.
Solution activity was maintained between 90-100%, temperature (+/-2F) and pH (+/- 0.1 units) using constant monitoring.
Standard Testing Procedure
The following procedure was followed for each of the 160 plus experiments and was designed to maintain constant workload throughout the test. The only time this procedure was not followed was for evaluation of workload effects.
1. Plate bolt for approximately 60 min to 0.4 mil along with a 1 × 0.75-inch pit panel.
2. After plating, remove bolt for ferroxyl evaluation, replace with a new 1 × 0.75-inch plating rate panel (to maintain workload) and continue plating pit panel to 2 mil.
3. Remove rate panel after 30 min and replace with dummy bolt; determine rate of deposition by XRF.
4. Remove dummy bolt and pit panel after 2 mil is deposited.
Results
A. Standardization of substrate effects and pretreatment for ferroxyl and pitting tests
The purpose of these 12 experiments was to uncover any substrate defects or pretreatment conditions that would contribute to inaccurate results during ferroxyl and pit evaluation.
The heavy metal stabilizer was modified to determine consistency of results over a range of plating conditions. The experimental matrix and results are tabulated in Table I.
Although the statistical sample is small, the results do indicate that the conditions of our experiment are repeatable and should not be subject to variations due to pretreatment and substrate effects. This creates a solid foundation for data evaluation based solely on the variables we were monitoring.
During each of four sets of experiments six critical variables were monitored: porosity, pitting, passivity, plating rate, pullback and plate-out.
B. Effect of solution age
An 800-ml high-phosphorus solution was operated out to 5 metal turnovers. Filtration through a 1-micron filter was performed at each metal turnover. Plating rate, deposit porosity, pitting and passivity were monitored. In addition, solution stability and edge effect were evaluated and levels of hypophosphite and orthophosphite recorded.
There were not many surprises here. Nickel metal and sodium hypophosphite levels were maintained near optimum. Although inconsistent from turnover to turnover, the increase in orthophosphite concentration is in agreement with published results, and the specific gravity increased linearly with solution age. As expected, the plating rate did decline and is due primarily to the increased level of orthophosphite,28 although the higher levels of complexor required to maintain phosphite tolerance also played a role. All panels maintained a semi-bright, clear appearance throughout the 5 metal turnover life test.
The stabilizer concentration remained consistent, which allowed us to accurately evaluate the effect bath age had on pitting and porosity independent of heavy metal stabilizer level.
Initial passivity data vs. solution life agreed with previous studies, indicating that passivity increased after the first 0.5 metal turnovers and then slowly declined with solution age.29
In agreement with several studies, porosity increased with bath age.30,31 Although the test was stopped at 5 metal turnovers, the continued increase in porosity with solution age is expected to continue. Deposit pitting did not appear to be solution age related, at least up to 5 metal turnovers.
Slightly more plate-out was evident as the solution aged. Besides the obvious introduction of shop dust and metal fines, some postulate that decreased stability with increasing solution age is due to the presence of insoluble nickel phosphide and/or gelatinous metal hydroxides that can cause localized reductions of nickel ions.26 The buildup of "micro" gelatinous metal hydroxides is most likely from dissolution of substrates during the initial displacement reaction. Phosphite intolerance and precipitation as the solution ages are other likely contributors to instability. 32
C. Effect of heavy metal stabilizer level
Four high-phosphorus solutions were prepared and plated to 0.5 metal turnovers. A heavy metal stabilizer was added to each of the solutions in varying amounts and the effects summarized in Table III. The testing parameters were as follows:
| Solution pH | 4.8 |
Temperature (F) |
190 |
| Agitation | 1-inch stir bar set at 5 (560 rpm) |
| Loading | 0.4 sq ft/gal |
| Stabilizer level (ppm) | variable |
The results in Table III clearly indicate a correlation between heavy metal stabilizer level, pitting and porosity. All other variables held constant, higher concentrations of heavy metal stabilizers promote higher porosity and pitting. Passivity was also inversely related to stabilizer level. The time to black was cut nearly in half with higher stabilizer levels.
Plating rate was independent of stabilizer level in the range tested. Edge pullback was encountered at 0.6- and 1.0-ppm levels. This is a common stabilizer level for many commercial high-phosphorus systems and may account for the "break in" period required for some of them. Although this was not tested and/or confirmed during our experimental matrix, the stabilizer concentration has been found to be less critical in terms of edge pullback as the solution ages.
Palladium stability increased with an increase in stabilizer level. Little plate-out was observed; however, the group of experiments above was not run during an extended period of time and the opportunity for plate-out to initiate was minimized.
D. Effect of workload to solution volume
Four high-phosphorus solutions were prepared and plated to 0.5 metal turnovers. All variables were held constant except various workload to solution volumes were evaluated. The results are summarized in Table IV. The plating parameters were as follows:
| Solution pH | 4.8 |
Temperature (F) |
190 |
| Agitation | 1-inch stir bar set at 5 (560 rpm) |
| Loading | variable |
| Stabilizer level (ppm) | 0.3 |
The results do show a causal effect on loading and pitting. Higher loading appeared to substantially reduce pitting. An explanation for this phenomenon is most likely that the heavy metal stabilizer was co-deposited uniformly and distributed over a larger surface area. This same thinking applies to the deposit passivity that showed a similar improvement as the workload increased. The same effect might have been expected for porosity, but that was not the case. Further studies may be necessary to determine the reasons for this.
Plating rate was unaffected by workload to solution volume, which contradicts other studies.24,25,26 This did not surprise us, however, since solution chemistry was maintained at or near optimum for the entire test. Previous researchers allowed the reactants to deplete well below recognized operating levels.
E. Effect of solution agitation
Five high-phosphorus solutions were prepared and plated to 0.5 metal turnovers. Variable types and amounts of agitation were introduced into the solution. The results are summarized in Table V. The plating parameters were as follows:
| Solution pH | 4.8 |
Temperature (F) |
190 |
| Agitation | variable |
| Loading | 0.4 ft2/gal |
| Stabilizer level (ppm) | 0.3 |
At first glance, the results appeared random and insignificant. Upon closer examination, the data does indicate a pattern. No agitation provided the best results for porosity and the worst for pitting. Based on this, as well as the data generated from our workload experiment, it appears there is a distinctly different mechanism that controls pitting and porosity. Low porosity deposits under conditions of no agitation support earlier claims that adsorption and co-deposition of stabilizers are diffusion controlled: less agitation, less adsorption, less porosity. When agitated, all porosity patterns disappear with no clear benefit derived from any type or amount.
It appears that interdependence exists between pitting and agitation. Higher agitation, regardless of type or amount, promotes lower pitting. This is in agreement with many of the EN troubleshooting guides.33
Passivity was affected by agitation. No agitation and moderate nitrogen produced the best results. Excessive agitation produced the worst. Again, this appears to be a diffusion and distribution phenomena where high agitation, especially rotational, promotes high adsorption of stabilizer into the deposit. Although the data points are limited, higher agitation did tend to promote higher plating speeds.
The average plating rate for the 11 experiments in this set (not including excessive and air) was 0.516 mil/hr. The plating rate increase for excessive agitation and moderate air is 16% and 18% respectively, well above our margin of error.
A marked improvement in solution stability was observed with an increase in agitation, regardless of type. This is in agreement with field observations that show a noteworthy decrease in equipment plate-out when solution movement is increased by various means.
Synergy
Until now, all experiments were conducted with only one operating parameter varied. EN operating conditions in the real world are not nearly as static. Optimum performance of an EN process is often a direct result of combining ideal operating conditions. The next group of approximately 120 tests evaluated the synergistic effect of loading, agitation and stabilizer concentration on deposit porosity and pitting.
Each group is comprised of 16 experiments where one parameter, such as solution loading, was held constant while all others were varied. For simplicity sake, the entire experimental matrix and results were tabulated and can be found as Table VI.
Due to the volume of data, operating trends were difficult to ascertain from Table VI. For this reason, an ascending sort was executed for the results of both deposit porosity and pitting. This simplified the data analysis and permitted easier recognition of the effects a particular parameter had on the results. Sorted porosity and pitting data can be found as Tables VII and VIII respectively.
Analysis of Synergy Experiment Results
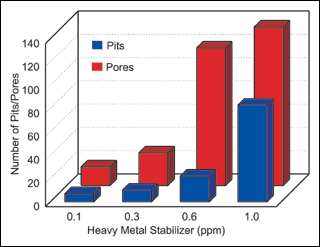
The first group of Heavy metal stabilizers.experiments compared the effect heavy metal stabilizers had on porosity and pitting. Again, due to the volume of data, the average of all experimental results were used and the results presented in Figure 1.
Figure 1 illustrates the effect that heavy metal stabilizer levels have on both porosity and pitting. During the sequence of 120 synergy experiments, the results clearly indicate a direct relationship between increased stabilizer level and increased deposit pitting and porosity. This relationship held independent of workload and agitation.
In Table VII, ascending sort of porosity data, 13 of the 16 experiments using the lowest level of heavy metal stabilizer (0.1 ppm) were found in the top one-third of the table. The only time a higher stabilizer level produced low porosity results was with higher workloads and moderate nitrogen agitation (exp. No. 7). This is in agreement with our results in Table II, which indicated higher loading reduced porosity.
In Table VIII, ascending sort of pitting data, 12 of the 16 experiments using the lowest level of heavy metal stabilizer were also found at the top 1/3 of the table. Again, the only time that higher stabilizer concentrations produced lower pitting was with increased workloads. (See experiments No. 66, 68, 70, 77 and 85 in Table VIII).
Of the 16 experiments run at the highest stabilizer level (1.0 ppm), 15 experienced edge pullback. (See Table V, exp. No. 49-64). It is interesting to note that of the experiments that resulted in edge pullback, a number of them had little or no pitting and very little porosity (See Table, exp. No. 66-70). This was surprising considering that pitting is often a pre-cursor to step plating and edge pullback. A review of the data did not yield a possible mechanism for this.
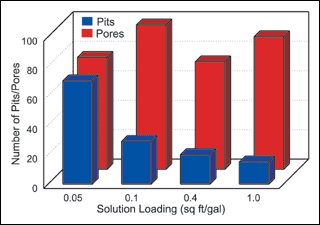
Solution loading. The second group of synergy experiments compared the effect of solution loading on deposit porosity and pitting. Again, an average of all experimental results were used, and the results presented in Figure 2.
Figure 2 illustrates the effect that workload to solution volume has on both porosity and pitting over a range of experimental conditions. The results are in agreement with earlier tests that show a decrease in deposit pitting with an increase in loading.The results of our synergy experiments are similar to earlier trials that indicated no real consistent effect of solution loading on deposit porosity.
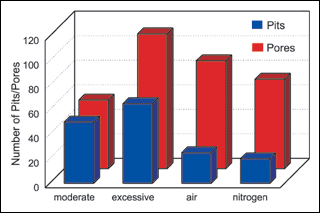
Solution agitation. The third group of synergy experiments compared the effect of solution agitation on deposit porosity and pitting. Averages of all experimental results were used and the results presented in Figure 3.
Figure 3 illustrates the effect that agitation has on both porosity and pitting over a range of experimental conditions. Overall, pitting was reduced when air or nitrogen agitation was introduced. A comparison of Exp. No. 32 with No. 25 and No. 27 in Table V highlights our findings. All three experiments were run under identical conditions except agitation was varied. Similar patterns can be found throughout the data.
By comparison, excessive rotational agitation (900 rpm) resulted in a 50-fold increase in pitting. This may be explained by the uniformity of high-speed rotational agitation. A vortex develops in the solution, creating a uniform flow pattern. This pattern of solution flow may deposit stabilizers onto fixed sites resulting in high pit areas. This may also explain the high incidence of skip plating under excessive agitation conditions. In contrast to rotational agitation, air or nitrogen agitation is not homogenous. The panels and bolts are not held in place as they are by the vortex and solution movement is irregular. This type of agitation appears beneficial to pit reduction and may do so by facilitating release of hydrogen gas bubbles.
Porosity also increased with agitation. A comparison of the results in Table V from experiments Nos. 2, 3 and 4 with experiment No. 16 shows a dramatic increase in porosity with an increase in rotational agitation. This phenomenon is exaggerated with low solution loading. These results are in agreement with earlier tests that show an increase in deposit porosity with an increase in agitation (see Table III).
Suggestions
1. Pay close attention to solution loading and make efforts to operate within thespecified process guidelines.
2. When operating below minimum loading recommendations, reduce all types of solution agitation and operate the solution chemistry below 85% activity (this reduces stabilizer levels). Monitor adverse effect on equipment plate-out.
3. Parts requiring optimum corrosion resistance should be plated between 0.2 (break in period) and 5.0 metal turnovers.
4. For heavy build applications (2-50 mils), operate the solution chemistry below 85% activity and use air agitation to minimize pitting.
5. If experiencing stability problems, maintain chemistry at or near optimum, increase solution agitation and introduce air if not already present.
6. If experiencing edge effect problems, operate at lower solution activity and reduce all types of agitation. Increase workload if possible.
7. To increase plating speeds, increase agitation. Monitor for edge effect problems.
8. If experiencing poor corrosion resistance due to high porosity, reduce agitation and operate solution below 85% activity.
9. If experiencing poor passivity, make sure plating speed is at or below 0.5 mils/hr, reduce agitation, increase workload and operate chemistry below 85% activity.
10. Work closely with your supplier.
11. Ask your supplier about adjusting an existing process or developing one specifically suited for your unique application or operating conditions.