Gold's Many Uses
Over many years, AESF/NASF stalwart Jack Dini contributed a series of fascinating columns to Plating & Surface Finishing, under the title Fact or Fiction?. What follows is another welcome addition to his output for the NASF. Here, Jack discusses the many uses for gold, some involving surface finishing, some not.
#nasf
by
Jack Dini
Featured Content
Livermore, California, USA
Editor's Note: Over many years, well-regarded AESF/NASF contributor Jack Dini contributed a series of fascinating columns to Plating & Surface Finishing, under the title Fact or Fiction?. The year 2020 saw a return of Jack’s writings from time to time. Here, we are happy to offer a yet another contribution to his output for the NASF. A printable version of this article can be accessed HERE.

by James St John, Wikipedia cc by 2.0
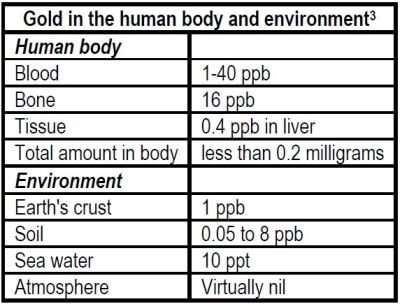
Gold is the most useful of all the minerals mined from the Earth. Its usefulness is derived from a diversity of special properties. Gold conducts electricity, does not tarnish, is very easy to work, can be drawn into wire, can be hammered into thin sheets, alloys with many other metals, can be melted and cast into highly detailed shapes, and has a wonderful color and brilliant luster. Gold is a memorable metal that occupies a special place in the human mind.1
The late Al Weisberg, former President of Technic Incorporated, provides this excellent introduction. “Gold is electroplated not only for psychological effects, but because of its unique combination of physical properties. Mythology, folk tales, the Bible and other written and oral records from all ages attest to gold's influence on the human animal. It has inspired greed, covetousness, and ostentation. It has been the instigator of war, rape, and murder. At the same time, it symbolically represents the sun, life, eternity, solidity, ultimate value and love. Although many countries have restrictions on the ownership of gold, it pervades our minds and lives. Gold is mentioned over 400 times in the Bible and nearly 300 times by both Shakespeare and Tennyson. It is a family name in many countries of both the East and West."2
Electroplating allows a small amount of the metal to be thinly and evenly distributed over a very large surface area so that it may simulate bulk gold and as such, be used to decorate a variety of less expensive consumer items. In the technical field, gold is electrodeposited in order to make use of its excellent characteristics in terms primarily of electrical, chemical and optical properties. Electrodeposited gold has satisfied many of the demands of the electronics industry.
A listing of the more important uses of gold includes the following:
- Jewelry
- Financial
- Electronics
- Aerospace
- Medicine
- Dental
- Glass
- Gilding
- Awards
These will be covered in this report along with some comments on the movie Goldfinger and gold facial treatments.
Jewelry
Jewelry is the primary use of gold making up to about 78 percent of its use on a yearly basis. Goldsmithing is still basically a craft industry, although the making of some items such as gold chains is now done mechanically. Pforzheim, Germany (Golden City), is a major center for jewelry with 11,000 people there being employed in the trade.
Financial
Because gold is highly valued and in very limited supply, it has long been used as a medium of exchange or money. The consumption of gold in investments is around 40%.4
Electronics

Wikipedia, Creative Commons CC0 1.0 Universal Public Domain Dedication
The most important industrial use of gold is in the manufacture of electronics. Solid state electronic devices use very low voltages and currents which are easily interrupted by corrosion or tarnish at the contact points. Gold is the highly efficient conductor that can carry these tiny currents and remain free of corrosion. Electronic components made with gold are highly reliable. Gold is used in connectors, switch and relay contacts, soldered joints, connecting wires and connection strips.
A small amount of gold used in almost every sophisticated electronic device. This includes: cell phones, calculators, personal digital assistants, global positioning system units and other small electronic devices. Most large electronic appliances such as television sets also contain gold.
One challenge with the use of gold in very small quantities in very small devices is loss of the metal from society. Nearly one billion cell phones are produced each year and most of them contain about fifty cents worth of gold. Their average lifetime is under two years and very few are currently recycled. Although the amount of gold is small in each device, their enormous numbers translate into a lot of unrecycled gold.1
Aerospace

Wikipedia, Public Domain
If you are going to spend billions of dollars on a vehicle that when launched will travel on a voyage where the possibility of lubrication, maintenance and repair is absolutely zero, then building it with extremely dependable materials is essential. This is exactly why gold is used in hundreds of ways in every space vehicle that NASA launches.
Gold is used in circuitry because it is a dependable conductor and connector. In addition, many parts of every space vehicle are fitted with a gold-coated polyester film which reflects infrared radiation and helps stabilize the temperature of the spacecraft. Without this coating, dark colored parts of the spacecraft would absorb significant amounts of heat.
Gold is also used as a lubricant between mechanical parts. In the vacuum of space, organic lubricants would volatilize and they would be broken down by the intense radiation beyond Earth's atmosphere.1
Medicine
Wikipedia, by Ragesoss, cc by SA 3.0
Gold has been of medicinal interest almost since its discovery. Unfortunately, most claims for its efficacy were unfounded and it was not until fairly recently that genuinely useful preparations finally appeared. Compounds of gold are now widely used in the treatment of rheumatoid arthritis, and now second generation drugs are appearing.
Gold is part of the medical armory against rheumatoid arthritis, and treatment with it even merits a special name, chrysotherapy, from the Greek word for gold, chrysos. It is prescribed when treatment with non-steroid anti-inflammatory drugs is failing to give relief.3
Gold is used as a drug to treat a small number of medical conditions. Injections of weak solutions of sodium aurothiomalate or aurothioglucose are sometimes used to treat rheumatoid arthritis.4
Small amounts of gold are used to remedy a condition known as lagophthalmos, which is an inability of a person to close their eyes completely. This condition is treated by implanting small amounts of gold in the upper eyelid. The implanted gold 'weights' the eyelid, and the force of gravity helps the eyelid close fully.
Radioactive gold is used in diagnosis. It is injected in a colloidal solution that can be tracked as a beta emitter as it passes through the body. Many surgical instruments, electronic equipment and life-support devices are made using small amounts of gold. Gold is nonreactive in the instruments and is highly reliable in the electronic equipment and life-support devices.1
Nanomedicine
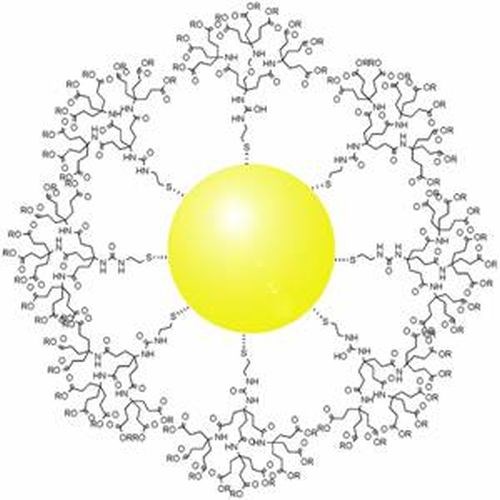
Wikipedia, Public Domain
Gold is an excellent candidate for nanomedicine because of its particular physico-chemical properties. Well tolerated by the body and easily malleable, this metal has, for instance, the particularity of absorbing light and then releasing heat, a property that can be exploited in oncology. Gold nanoparticles (small particles that range in size between 1 to 100 nanometers) can be used to target tumors. When exposed to a light source, the nanoparticles release heat and destroy neighboring cancer cells.
By testing a variety of gold nanoparticles, researchers are providing first evidence of their impact upon human B lymphocytes - the immune cells responsible for antibody production. The use of these nanoparticles is expected to improve the efficacy of pharmaceutical products while limiting potential adverse effects. These results will lead to the development of more targeted and better tolerated therapies, particularly in the field of oncology.5
Oncologists are now injecting cancer patients with ultra-tiny, gold wrapped spheres. The nanoparticles, each smaller than a red blood cell, accumulate in a tumor after slipping out of the blood stream through little holes in the tumor's rapidly growing vessels. Once there, the gold waits until an oncologist blasts it with near-infrared light which converts the light into heat, and as temperatures in the tumor climb above 104°F, the cancer cells deform, shrivel and then disintegrate.6
Dental
Dentists have been using gold since the time of the Etruscans, who lived in Italy from 1,000 to 400 BC, where excavated graves have revealed skulls with false teeth held in place by gold bridges. Today, more than 60 tons a year of gold is used in dentistry. Gold fillings, crowns and teeth are particularly popular in Germany and Japan. Dental gold is alloyed with silver and palladium, plus a trace of zinc (0.2%), to harden it, with the proportion of gold being around 75%.3
Glass
Gold has many uses in the production of glass. The most basic use in glass making is that of a pigment . A small amount of gold, if suspended in the glass when it is annealed, will produce a rich ruby color.
Gold is also used when making specialty glass for climate-controlled buildings. A small amount of gold dispersed within the glass or coated onto the glass surface will reflect solar radiation outward, helping the building stay cool in the summer, and reflect internal heat inward, helping them stay warm in winter.
The visor on the helmet of an astronaut's space suit is coated with a very thin film of gold. This film reflects much of the very intense solar radiation of space, protecting the astronaut's eyes and skin.
Gold gilding and gold leaf
Gold has the highest malleability of any metal. This enables gold to be beaten into sheets that are only a few millionths of an inch thick. These thin sheets, known as 'gold leaf' can be applied over the irregular surfaces of picture frames, molding, or furniture.
One of the most eye-catching uses of gold leaf is on the domes of religious buildings and other important structures. Gold brush plating has also been used on domes.
Oscars

Wikipedia, Fair Use
Oscar and Emmy award trophies have been manufactured at R. S. Owens & Company in Chicago. The company produces approximately 50 Oscar trophies each year. The award weight 8.5 pounds and is 13.5 inches tall.
They are made of britannium, an alloy of 92% tin, 2% copper, and 6% antimony and plated with four metals. First the statues receive a copper coating, then nickel, then silver followed by 24 karat gold.
Goldfinger”

Wikipedia, Fair Use
Though only a brief scene, the 14-karat demise of “Bond Girl' Shirley Eaton remains the movie Goldfinger's most iconic image. “She died of skin suffocation,” 007 tells M. “It's been known to happen to cabaret dancers. It's all right so long as you leave a small bare patch at the base of the spine to allow the skin to breathe.”
James Bond was a much better agent than a scientist. We breathe through our noses and mouths, not our skin (though clogging the pores for an extended period can cause heat-stroke). The filmmakers, however, believed author Ian Fleming's death by gold scenario was a genuine risk and took precautions. A physician was present during the filming, and after filming the make-up was removed as quickly as possible. Eaton, now 84, said her experience with body paint hasn't affected her fondness for gold. “I wear it often,” she says. “I have always liked gold as a metal for its warmth.”7
Facial treatments
Gold facial treatments allegedly provide a number of benefits including better blood circulation, wrinkle removal, anti-aging and on and on.8 These aren't cheap, and you should expect to pay anywhere from $100 to $300 for a full-price gold facial.
References
1. Hobart M. King, “The many uses of gold,” geology.com (accessed July 17, 2020).
2. A.M. Weisberg, “Why Use gold?”, in Gold Plating Technology, Frank H. Reid and William Goldie, Editors, (Ayr, Scotland, Electrochemical Publications Ltd., 1974); p. 12.
3. John Emsley, Nature's Building Blocks, (Oxford University Press, 2001).
4. “Gold,” en.m.wikipedia.com, (accessed December 15, 2020).
5. “Medicines made of solid gold to help the immune system,” Science Daily, June 28, 2019.
6. Marissa Fessenden, “A golden cure?”, Smithsonian, February 2014.
7. “Gold-blooded murder,” Smithsonian, February 2014.
8. Keith Flanagan, “7 gold infused spa treatments that will make you look like a million bucks,” robbreport.com, October 15, 2017.
About the author

Jack Dini earned a Bachelor of Metallurgical Engineering degree from Cleveland State University and began his career in the 1950s with Cleveland Supply Co. (now Pavco). He spent a few years at Republic Steel's research center and Battelle Columbus Laboratories. In 1962, he joined Sandia Laboratories, Livermore, CA, where he was involved with electrodeposition projects for 18 years before moving to Lawrence Livermore (LLNL) in 1980. He was section leader, fabrication processes. Responsibilities included direction of activities in five groups: electroplating and metal finishing, vacuum processes, metal fabrication, plastics and optics.
Mr. Dini is a prolific scientist. He is the author or coauthor of some 125 technical papers and, while many researchers are content to specialize in one or two fields, he made significant contributions to more than half a dozen disciplines in surface finishing. He is the author of two books, Electrodeposition- The Materials Science of Coatings and Substrates, and Challenging Environmental Mythology: Wrestling Zeus. The scientific community is fortunate that he carefully documented his work, sharing it with others around the world. It includes plating uncommon metals, alloy plating, printed circuits, chemical milling, electrojoining and gathering electrochemical/property data.
RELATED CONTENT
-
Qualitative Approach to Pulse Plating
In 1986, the AESF published Theory and Practice of Pulse Plating, edited by Jean Claude Puippe and Frank Leaman, the world’s first textbook on pulse plating. A compendium of chapters written by experts in this then-emerging field, the book quickly became the authoritative text in pulse plating. What follows here is the opening chapter, serving as an introduction to the field. Although the field has grown immensely in the intervening 35 years, the reader will find that the material remains a valuable introduction to those looking to advance the field of pulse plating.
-
A Process for Alkaline Non-cyanide Silver Plating for Direct Plating on Copper, Copper Alloys and Nickel Without a Silver Strike Bath
Traditionally, silver is electroplated in toxic, cyanide-based chemistry. Due to cyanide’s extreme hazard to human health and environments, developing non-cyanide silver chemistry is essential for the silver electroplating industry. Discussed here is an aqueous, alkaline non-cyanide silver plating technology, which can be directly plated over nickel as well as copper and its alloys. The silver deposits have perfect white color and better anti-tarnishing properties than other non-cyanide silver processes. The silver is plated entirely from the dissolving silver anode and the bath is very stable, and maintains a stable pH level both during plating and idle time. This new non-cyanide silver technology will plate bright silver that is perfectly suitable for electronic, industrial and decorative applications. .
-
AES Research Project #41: Part 4: Adhesion Failure of Electrodeposited Coatings on Anodized Aluminum Alloys
An SEM study of peel-test adhesion specimens from plated coatings on anodized aluminum shows that failure can be categorized in three different modes: (1) specimens exhibiting poor adhesion strength, which fail at the anodic film/coating interface; (2) specimens with good adhesion strength, which fail by local fracture of the anodic film and (3) specimens with excellent adhesion strength , which fail when the applied load is greater than the strength of the alloy substrate. The effect of anodizing parameters and alloy composition on peel test failure are discussed.


















