Greener Hard Chromium Plating
By improving tribological properties as well as corrosion resistance, hard chromium plating can lower energy consumption of moving parts and machinery, reduce the need to replace parts frequently, leading to reduced waste and improved efficiency.
#research #energy #surfin
(A printable PDF version of this paper is available by clicking HERE.)
ABSTRACT
Featured Content
Hard chromium plating has been in commercial use since the 1920s and has had a major positive impact on society. It is commonly used to improve the properties of ordinary materials so that they can be applied in areas where they would not normally have been suitable. By improving tribological properties as well as corrosion resistance, hard chromium plating can lower energy consumption of moving parts and machinery, reduce the need to replace parts frequently, leading to reduced waste and improved efficiency. Unfortunately, the hard chromium plating process itself involves the use of highly toxic materials and, due to its low efficiency, generates harmful mist through the electrolysis of water. This paper will describe methods and technologies that are being implemented to make hard chromium plating “greener.”
Keywords: hard chromium plating, environmental compatibility, green chemistry
Introduction
The hard chromium plating process is a very mature technology and has been in commercial use since the 1920s. Hard chromium plating has brought many benefits to society since its inception. It is typically used to improve the capabilities of ordinary materials so that they can be used for functions where they would not normally be suited. Hard chromium plated objects, like those shown in Fig. 1 below, generally have significantly better tribological attributes compared to the base material alone, not to mention better corrosion resistance. Examples of the benefits a hard chromium coating gives to a part are:
- Greater hardness
- Lower coefficient of friction
- Improved wear resistance
- Higher galling resistance
- Chemical inertness
- Superior corrosion resistance
These imparted improvements have been beneficial to society by helping to reduce energy consumption, reduce consumption of resources and reduce the amount of waste generated, not to mention its aesthetic appeal. Unfortunately, the hard chromium plating process itself involves the use of highly toxic substances, such as hexavalent chromium compounds and lead compounds.

Figure 1 - Examples of hard chromium plated parts.
Green Issues with Hard Chromium Plating
Use of hazardous substances
Hard chromium plating involves the use of hazardous substances. Current hard chromium plating processes are predominantly based on chromic acid and sulfuric acid and mainly employ the use of lead/tin alloy anodes. During plating, a lead oxide layer is formed on the surface of active anodes and, when they become passive, or plating stops, a layer of lead chromate is formed which generally falls from the anode, forming a sludge on the bottom of the plating tank. Additionally, some processes still use fluoride-based secondary catalysts. For suppressing the amount of mist generated during plating, perfluorooctane sulfonate (PFOS)-based mist suppressants have been commonly used. All of these substances are classed as hazardous. Figure 2 lists typical substances found in hard chromium plating baths and their associated hazard symbols.
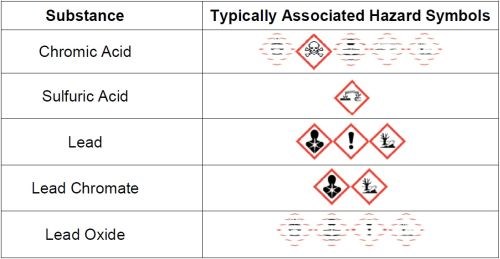
Figure 2 - Typical substances and their hazard symbols.
Hexavalent chromium is a well-established occupational carcinogen,1,2 as well as being a highly oxidizing and corrosive chemical. The hazardous nature of hexavalent chromium means that several of its substances are currently included in annex XIV of The EU REACH legislation.3 The use of these substances will require specific authorization before the sunset date which is on 21 September, 2017. Hexavalent chromium substances and their use are strictly controlled with tight emissions limits and exposure limits being enforced.
Lead and its compounds are also known to be hazardous,4,5 and are also controlled. The USA controls the use of lead anodes for metal finishing applications through its Toxic Chemical Release Inventory (TRI),6 where metal finishing companies are required to register the use of lead at their facilities if they exchange at least 64 lb. (~29 kg) of lead anodes each year.
Exposure to hazardous substances
A person can be exposed to the hazardous substances used during hard chromium plating in various ways:
- Making additions to the plating bath
- Taking a sample of the bath for analysis
- Spills and leaks leading to contact with individual substances
- Mist generated during plating that can then be inhaled
- Contact with contaminated equipment surfaces
- Maintenance, cleaning and replacement of anodes
- Sludge removal from the bottom of the tank
- Maintenance or treatment of the plating solution
- Liner replacement
- Equipment maintenance.
Exposure can occur easily and, with the toxic nature of the main substance, hexavalent chromium means that engineering measures and personal protection is essential to ensure the health and safety of operators and maintenance personnel.
Emissions of hazardous substances
Hazardous substances from hard chromium plating can be emitted, as solids, liquids, dust or aerosols into the local or wider environment in various ways, amongst which are:
- Mist generated during plating
- Drag-out of the plating solution
- Residues in containers used for the various chemicals
- Cleaning of the line
- Anode replacement
- Anode cleaning
- Sludge removal
- Tank liner or rack coating replacement
- Equipment maintenance or parts replacement.
Due to these circumstances, care has to be taken to prevent or minimize the emissions of hazardous substances and to ensure that they are properly controlled and the associated wastes correctly handled and disposed of.
Resource wastage
Hard chromium plating processes generally have current efficiencies in the range of 10 to 30%. This means that, for even the most efficient processes, at least 70% of the electrical energy is wasted, in that it is not being used for plating chromium metal. This is a serious waste of energy meaning that high current densities are typically used for hard chromium plating compared to plating processes for other metals like nickel and copper.
The effect of this low efficiency is further compounded by the negative effect of metallic contamination, reducing the conductivity of the bath and resulting in higher voltages to drive the plating process. Metallic contamination can come from etching or dissolution of the parts being plated, from using non-DI water and also trivalent chromium, which is a side reaction of hard chromium plating as well as from other sources. Using plating baths with excessive amounts of metallic contamination not only compromises the quality of the plated deposit, potentially increasing the scrap rate of plated parts thus leading to waste, but also increases the amount of electrical energy required (kWh), due to the higher voltages needed compared to a less contaminated plating bath.
The correct arrangement of anodes and the racking of the part/s being plated are also important, as the wrong set-up can lead to excessive plating in high current density areas. This phenomenon is commonly referred to as “dog-boning” as illustrated in Fig. 3 below. This means that the distribution of plated chromium is not even across the part.
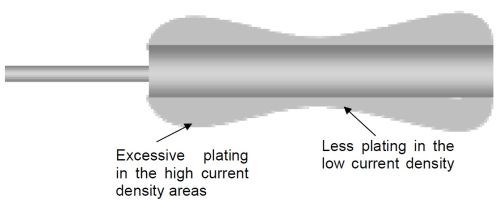
Figure 3 - Example of poor plating distribution ("dog-boning").
Having dog-boning generally requires that more chromium be plated on the part to ensure that a certain minimum thickness is achieved in the low current density areas. This not only wastes time but also resources, as more chromium metal and electrical energy is required than would normally be required if the plating distribution was more uniform. Metallic contamination of a plating bath exacerbates this effect and so the plating thickness difference in high current density areas compared to low current density areas increases the more contamination there is. Additionally this type of dog-bone plating typically requires some remedial action whereby the plated chromium is made level across the part. This typically involves the use of a post-grinding step to grind back the excessively plated areas so that the final chromium layer is even. This is a waste of resources due to the extra grinding step required that could have been avoided, not to mention the extra generation of waste coming from the grinding process.
Lead/tin anodes are constantly being eroded so have a limited lifetime. This leads to a need for replacements every year and consumes lead and tin resources as well as the resources required for transporting the anodes to the plating shop. This is a waste as there are insoluble anodes available that last significantly longer before needing to be replaced.
Plating solution can be lost through mist being sucked into the extraction system or by parts and racks dragging solution into rinses. Generally, only a small portion of this loss is returned to the plating bath, thus wasting good plating solution and leading to a higher consumption of plating chemistry. This consumes more resources for replenishment of the plating bath chemistry as well as resources needed to deal with the waste produced.
Replacing Hazardous Substances
Replacing hexavalent chromium
Hexavalent chromium substances used for hard chromium plating solutions are under a lot of pressure due to their highly toxic nature. A logical replacement for hexavalent chromium in hard chromium plating baths would be trivalent chromium. The expectation is that as the trivalent chromium process will also plate a chromium metal deposit then there should be little change required for current practices to be able convert to it.
Although there has been a lot of activity to find a replacement for the highly toxic hexavalent chromium substances in hard chromium plating baths there is currently no commercial trivalent chromium hard chromium process available. Successful trivalent chromium processes have been around for decorative chromium plating applications for decades but the step from a thin decorative coating to a thicker functional one is extremely difficult. There are non-chromium alternative processes available in the market that are used for specific wear resistant or corrosion resistant applications which are gaining popularity but none of them can cover the vast variety of applications that is currently covered by hexavalent hard chromium plating. It appears that the industry is still setting its hopes on a suitable trivalent chromium based alternative being released to market in the near future.
Replacing lead
Lead is a known hazardous substance and is controlled in many countries. Its use in hard chromium plating for anodes is based on its cheapness and resistance to chromic acid when alloyed with tin or similar metals. During plating the lead in the anodes normally generates a thin lead oxide layer on its surface. This is useful to the plating process in that it helps to more efficiently re-oxidize trivalent chromium formed at the cathode to hexavalent chromium.7 Lead oxide is, however, a hazardous substance. Another hazardous substance formed by lead anodes in hard chromium plating baths is lead chromate.
During normal plating practices the chromate slowly falls off the anodes and an orange sludge is formed on the bottom of the plating tank. This hazardous sludge is normally removed from the tank by hand. Lead anodes including the oxide and chromate coating are shown in Fig. 4.

Figure 4 - Lead/tin anodes including oxide and chromate layers.
Lead is also a soft metal. This softness means that anodes can easily deform or bend as shown in Fig. 5. With the anodes bending in this way, the geometry and spacing to the part being plated changes and this affects the current density distribution. For bent anodes, the current density would be different from top to bottom and thus the part would be plated thicker at the bottom compared to the top. In extreme cases, the anodes could make a short circuit when they become so bent that they finally touch the cathode (part).
Due to the soft nature of lead, such anodes need regular maintenance to ensure that the connections remain good and that bending like that shown in Fig. 5 is prevented (sometimes by regularly turning the anodes).

Figure 5 - Lead/tin anodes bent during plating.
Lead for hard chromium plating can be easily replaced.8 Platinized titanium (Pt/Ti) anodes have been effectively used for hard chromium plating for decades. Pt/Ti anodes generally use titanium or titanium-clad copper frameworks on which sheets of titanium expanded metal sheets covered in a thin layer of platinum are attached. The platinum stops titanium oxide from forming on the titanium metal surface which would reduce current flow and effectively stop plating from occurring. Titanium is used for the base material as it is stable against most, non-fluoride, hard chromium plating baths. Figure 6 shows examples of Pt/Ti anodes used in the market.
Pt/Ti anodes have the following technical benefits over lead anodes:
- Toxicity - they only contain titanium and platinum and not lead or its compounds.
- Lifetime - they generally last for years before needing to be recoated.
- Form stability - they retain their shape during usage.
- Ease of shaping - they can be formed into almost any shape.
- Weight - they generally weigh only about 5-10% that of lead anodes.
- Maintenance - low maintenance
- Dummying in - no dummying in of the anodes required
- Sludge - no sludge is formed as the anodes don’t dissolve like lead.

Figure 6 - Examples of platinized titanium anodes.
It should be noted, however, that Pt/Ti anodes are more expensive to purchase than Pb/Sn anodes, mainly due to the platinum metal cost, even though it is a very thin layer. However, their longer lifetimes and technical benefits can help to offset this extra cost. As there is no lead oxide film on the anode surface, the re-oxidation of trivalent chromium to hexavalent chromium is less effective and in some system set-ups, this can lead to a steady increase in the trivalent chromium content of the plating bath. Fortunately, there are several solutions available to combat this issue, as discussed later under the section on “Trivalent chromium control (TCC) for Pt/Ti anodes.”
Replacing the fluoride catalyst
Fluoride-based secondary catalysts for hard chromium have been around since the 1950s. Although the plating process has a better current efficiency and gives improved wear resistance compared to the conventional hard chromium process, fluoride is an extremely aggressive chemical, especially at the pH of <1 in hard chromium baths. The fluoride substances are not only hazardous, but the high chemical etching effect means that basis materials are heavily etched and attacked as well as the plating tank, liner, anodes and surrounding equipment. All of this means that fluoride-based plating baths generally become contaminated with metals quickly, requiring more frequent replacement and more waste results. Exposed areas of parts are attacked more when immersed in the plating bath, which then require extra protection to be fitted. Further, there is a higher part reject rate due to this attack, again leading to wasted effort and waste of resources.
In the 1980s, an organic high efficiency etch-free catalyst system was developed, which not only reduced the high etching effect inherent in fluoride-based processes but also managed to improve the efficiency of the process as well as the wear and corrosion resistance of the plated parts. This type of organic catalyst system can be used to replace the fluoride catalyst plating process and is the most common secondary catalyst type system used for hard chromium plating worldwide.
Figure 7 shows the loss of hard chromium from Taber panels plated using the three main hard chromium processes: organic catalyst, fluoride catalyst and conventional, after 10,000 cycles using a 1-kg force with CS-10 wheels. The plated layer from the organic catalyst process clearly shows considerably less weight reduction, indicating that the wear is significantly less than that for the deposits from the fluoride or conventional processes. This highlights one of the many technical benefits of the organic catalyst process versus the fluoride process, that of superior dry wear resistance.
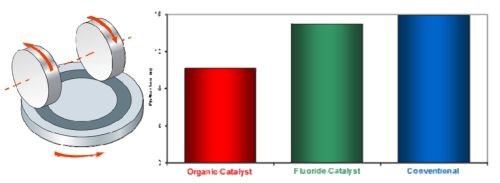
Figure 7 - Taber wear test results of hard chromium from different processes.
Replacing PFOS
Due to the low efficiency of the hard chromium plating processes, a large amount of the electrical energy goes into forming oxygen and hydrogen gas at the respective anodes. This manifests itself as little bubbles that rise quickly to the surface of the plating solution and then burst, sending small droplets of plating solution into the air. These droplets then form a toxic mist above the plating bath that, if not sufficiently extracted, can pollute the immediate surroundings and can therefore have serious health, safety and environmental (HSE) consequences.
A common method used for decades is to employ a surfactant that either forms a foam blanket on the surface of the plating bath to physically retard the droplets or to reduce the surface tension of the solution to reduce the size of bubbles and reduce the speed of droplets. Both outcomes can have the effect of reducing mist generated by over 90%. The second type of mist suppressants, called surfactant-based mist suppressants or wetting agent fume suppressants (WAFS), have also been developed so that the surface tension is reduced giving good mist suppression, but the foam on the surface of the bath is also kept at a low level. This development was brought about to reduce the risk of hydrogen explosions. The foam contains a perfect mix of hydrogen and oxygen and with the high amount of electrical energy used during hard chromium plating, there is the potential that a spark or a hot surface can act as an ignition source.
A common substance historically used for mist suppressants is perfluorooctane sulfonate (PFOS), shown in Fig. 8. PFOS is a very stable organic substance able to withstand the highly aggressive hard chromium plating chemistry. This stability is mainly due to the perfluorinated structure of the substance.
Figure 8 - Space fill structural model of perfluorooctane sulfonate (PFOS).
PFOS is classified as a persistent, bioaccumulative and toxic substance (PBT). The use of PFOS and its salts was banned in 2007 in the USA, in 2008 by the EU and in 2010 for most of the world through the UNEP Stockholm Convention on Persistent Organic Pollutants.9 There are and have been exemptions in place, including the hard chromium plating application, but these are regularly reviewed to determine if there are suitable alternative substances or technologies. The use of PFOS for hard chromium plating is banned in Japan10 and Canada.11 The United States Environmental Protection Agency plans to ban the use of PFOS for hexavalent chromium plating and anodizing applications after 21 September, 2015.12

Figure 9 - Examples of non-PFOS and non-PFC mist suppressants.
There have been alternative non-PFOS mist suppressant substances available for at least 20 years,13 mainly giving foam blanket protection. Low foaming versions of non-PFOS surface tension-reducing mist suppressants have also been available since 2010. A second generation of non-PFC (perfluorinated compound) and non-PFOS low-foaming mist suppressants have been available since early 2013. Figure 9 shows beakers containing hard chromium plating solutions during plating where different non-PFOS and non-PFC mist suppressants have been added.
Greener Equipment Developments
Conformal platinized titanium anodes
The technical benefits of Pt/Ti anodes compared to lead anodes were discussed earlier. Another feature of Pt/Ti anodes is that they are usually constructed from a titanium mesh. This mesh can be formed into almost any shape, remains stable in that shape and is open, allowing plating solution to pass through it. This makes it ideal for constructing conformal anodes that are specially shaped to match the shape of the part being plated. In this way the plating and thickness distribution of the plating all over the part can be optimized to reduce dog-boning and reduce the need for overplating and heavy post-grinding. It can also enable the application of higher current densities to improve productivity.
As hard chromium plating is commonly performed on cylindrical parts or parts with a simple geometry then the use of cylindrical, conformal anodes can give several advantages over the common system for lead anodes which typically consists of rows of anodes and parts. Figure 10 shows a typical arrangement for short rods or bars plated using “stick” lead anodes. The parts are racked in a row and situated in the middle of two rows of anodes. A representation of the plating distribution around the plated rod is shown by the red color. As would be expected there is more chromium plated in the areas nearest to the anodes.
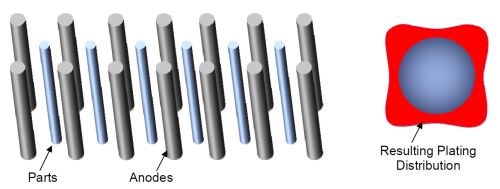
Figure 10 - Rows of anodes and cylindircal parts and the effect on plating distribution.
Rods plated in this way generally have to conform to certain specifications for concentricity or circularity so they normally need to be ground to level off the plated chromium and to achieve the required circularity. This means that a large portion of the chromium has to be ground away until the part is suitably rounded. Also such parts normally require a minimum thickness of chromium plating. This means that the total amount of plating needed on the part is dependent on the minimum plated thickness achieved. This means that a large excess of chromium is plated on the part to ensure that after grinding this minimum thickness can be achieved. This is a waste of resources and energy due to the extra plating required that will be simply ground away. Further, an extra grinding step is required to get the parts round again.
If conformal Pt/Ti anodes are used, then the anode/cathode arrangement changes as shown in Fig. 11 and the resulting plating distribution is more uniform around the rod.
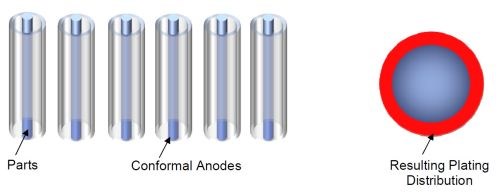
Figure 11 - Cylindrical conformal anodes and parts and the effect on plating distribution.
This means that savings can be made in the energy used and resources required, as parts can be plated quicker, and to size, reducing the amount of overplated chromium and reducing the amount of post-grinding required, if indeed post-grinding is actually needed.
Combined with a suitable masking system and equipment set-up to avoid dog-boning top to bottom on the rod means that the chromium can be effectively plated to size so that the plated rods only need post-finishing and the grinding step can be removed altogether. This reduces resource waste as less chromium is plated, less electricity is used and the grinding step is completely avoided. This of course gives financial benefits as well.
Water and plating solution recycling concepts
A simple hard chromium plating operation has some form of pre-treatment for the parts being plated, anodic etching and hard chromium plating in the same or different tanks and then rinsing. The anodic etch and hard chromium tanks typically employ some form of extraction system to remove the hexavalent chromium mist generated during plating. With this set-up, the hexavalent chromium-containing bath chemistry is dragged-out into the rinse or rinses which in turn need to be waste treated. The hexavalent chromium contained in the mist generated during plating is washed in the scrubber attached to the extraction system and the waste from this is then waste treated. Under such conditions, considerable chemistry is wasted due to drag-out and misting.
There is the technique employed where a drag-out rinse is installed before the main rinses themselves. The contents of this drag-out rinse can be used to top up the hard chromium or anodic etch plating tank, thus reducing some of the chemical wastage.
There are even more elegant water recycling concepts and systems in operation where only a minimal amount of DI water enters the complete plating system and only a small amount of hard chromium solution leaves the system. The rest is re-used in one way or another, for top-ups etc., but remains within the plating system.
By employing such concepts, the amount of water and chemical additions is greatly reduced and the amount of waste generated is also significantly reduced. This reduces waste of resources and can also save money.
Plating line housing
In addition to the typical extraction systems and other HSE measures for protecting members of staff from harm associated with hard chromium plating lines, the use an enclosure of the line means that this protection can be increased. An example of a housed hard chromium plating line is shown in Fig. 12.
The housing helps to isolate the heart of the plating line from direct access by personnel. This helps to protect them from the typical physical dangers of the process and equipment itself as it is a physical barrier. By implementing the necessary safety protocols and installing suitable safety equipment on such a housing means that unwarranted access can also be prevented, further protecting people from harm.

Figure 12 - A high productivity hard chromium plating line within a housing.
By utilizing housing technology and implementing relevant safety features means that the risk of harm to people from hard chromium plating process can be reduced and more tightly controlled.
Pumped dosing of liquid additives
As with all plating processes, the various additives and the source of chromium metal have to be regularly replaced to keep the process in good working order. Typically solid chromic acid-based products have been used. This can be a very dusty operation and can be physically demanding, as mostly 100-lb. or 50-kg containers are used. The dust contains a high concentration of hexavalent chromium and is a major source of the hazardous substance emissions, requiring the person making the addition to wear suitable personal protective equipment (PPE) and other precautions to be taken.
For many years, liquid additives have been on the market to minimize emissions and to avoid hexavalent chromium dust. This is a good step in reducing risk of harm to people during the addition process.
A further development is to install pumped dosing stations for the liquid additives like the one shown in Fig. 13. This means that larger containers can be utilized, meaning they need to be changed less often, reducing personal exposure. Also the liquid is pumped to the required tanks, meaning that additions don’t have to be done by hand. This further reduces exposure.

Figure 13 - Liquid hexavalent chromium additive pumping station.
By connecting some form of dosing control, whether by use of a simple ampere-hour meter or through the sophisticated software system used to control the plating line, the additions of additives can be made more regularly and can be more controlled. This gives the benefit of keeping the plating solution in a more steady state and reduces the chances of big swings in component content that could alter the effectiveness of the plating process. This can help to maintain constant, good quality of plating and reduce scrap parts being generated therefore reducing waste of resources.
Pumped sampling systems
Another source of exposure to hexavalent chromium is the taking of plating bath samples for laboratory analysis. This has to be done regularly and is normally performed by hand while the plating line is running. This can be a risk for staff tasked with doing this. By employing a pumped sampling system, samples can be taken from a location remote from the plating line itself, thus reducing risk of physical injury and exposure to hexavalent chromium mist. An example of a pumped sampling station is shown in Fig. 14.

Figure 14 - Pumped sampling station.
Improved ion exchange systems
In hard chromium plating, there is commonly a build-up of metallic contamination over time. This is mainly due to etched parts made of metals like steel and the subsequent build-up of iron contamination. To help to remove such contamination from hard chromium plating baths, a common technique is ion exchange. This removes contaminant metals, in the ionic forms, such as Fe, Cu, Ni, Zn, Cr+3, etc., while leaving the hexavalent chromium alone.
Once an ion exchange resin has become loaded with contaminants, it has to be regenerated. This requires washing it with sulfuric acid and then rinsing it thoroughly with DI water. For 500 liters of ion exchange resin in a standard ion exchange system this can result in up to 4,000 liters of wastewater per regeneration.
There are newer designs of ion exchange system that can drastically reduce the amount of wastewater generated, by as much as 50%. These newer systems also tend to incorporate extra safety devices so giving environmental as well as safety benefits. Such a newer design of ion exchange system can be seen in Fig. 15.

Figure 15 - A newer generation of an ion exchange system.
High productivity system for shock absorber rods
There are tens of millions of cars produced each year and even more are already on the roads. Each of these cars requires several shock absorbers. Due to the large numbers of shock absorbers required each year to fulfil this demand and the large variety of shock absorber types, a high capacity, highly versatile plating system has been developed to hard chromium plate the rods used in such shock absorbers.
This system utilizes several of the greener equipment developments mentioned in the previous few pages. By using an organic catalyst plating process at high current densities, conformal Pt/Ti anodes, adaptive shielding, automatic dosing and a housed line safety concept, the system has become the favorite choice for high volume shock absorber producers. The line can be integrated into the process flow from grinding lines through to baking and post-finishing and can virtually eliminate the need for post-grinding. All of this, combined with the housed line safety concept and integrated safety features, means that a producer can quickly plate a large variety of different rods, plated to size, thus removing the need for post-grinding. Further, the producer can better protect the workers against potential harm from the plating process.
This system can reduce the amount of chemistry required to produce the rods by at least 50% and reduce the electricity required by over 50%. These benefits, combined with the added benefit that a post-grinding step is generally not required, means a large reduction in resource usage and waste, as well as major process cost savings.
Continuous horizontal platers for hydraulic rods
Typically hydraulic rods are plated in large racks on conventional vertical hoist lines. A type of system specifically for this purpose that has been developed, using some of the greener equipment developments, is the continuous horizontal plating system. An example is shown in Fig. 16. The continuous horizontal platers feed the rods into the etch and plating cells of the equipment while turning them. By pumping a small amount of plating solution into the plating cells and by using conformal anodes, the rods are plated uniformly.

Figure 16 - A continuous horizontal plater for hydraulic rods.
The plating cells are enclosed, reducing mist emissions and exposure to people in the vicinity of the machines. The lines have a very small volume of hexavalent chromium chemistry and have very little solution drag-out. Combined with the superior plating performance and lower plating distribution variances in comparison to conventional lines, this means that wastage of resources is greatly reduced and protection of people and the environment can be improved.
Greener Process Developments
Trivalent chromium control (TCC) for Pt/Ti anodes
There is a recent development, called trivalent chromium control (TCC) technology, which is a plating process that contains a catalyst system that improves the re-oxidation efficiency of Pt/Ti anodes to such an extent that the issue of increasing trivalent chromium content can be reversed and controlled at a reasonably low level. This is non-lead technology, so the use of lead in the hard chromium plating process can be completely eliminated without having to dummy plate the bath regularly or use other methods such as ion exchange to control trivalent chromium content.
As TCC technology increases the efficiency of Pt/Ti anodes to re-oxidize trivalent chromium to hexavalent chromium, it doesn’t immediately reduce the level of trivalent chromium, as it requires a number of ampere-hours of plating to allow the anodes to do their work. Typically, a normal plating solution would be converted to a TCC plating solution through simple addition of the relevant TCC catalyst system. After slide converting the solution with TCC, a reduction in trivalent chromium content is seen over time, which then reaches an equilibrium level usually significantly lower than before.
Figure 17 illustrates such a conversion for a horizontal rotogravure process where Pt/Ti anodes were employed. The limited geometry of the system meant that the geometric anodic area was only marginally larger than that of the surface of the rotogravure rollers being plated. This meant that the re-oxidation of the Pt/Ti anodes being employed was not high enough to control the trivalent chromium to a low enough level for high quality plating. The plating bath was simply converted to a TCC bath and the process ran as before.
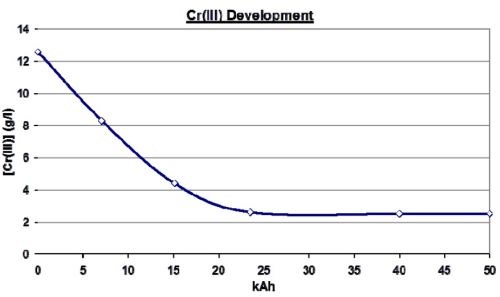
Figure 17 - Trivalent chromium reduction and control for a rotogravure application.
As can be seen, it took some time to re-oxidize the high content of trivalent chromium, but once that was done, the bath settled at an equilibrium of just under 3 g/L trivalent chromium instead of over 12 g/L trivalent chromium without the TCC technology.
Sulfuric acid-based, non-electrolytic, chromium strippers
Associated with hard chromium plating is the stripping of hard chromium. This is sometimes required because of the need to rework incorrectly plated parts or for reworking or refurbishing used parts. Typically, parts are stripped electrolytically in alkaline or acidic solutions or are immersed in acidic stripping solutions based on hydrochloric acid.
The immersion stripping in hydrochloric acid-based strippers is preferred in most cases, as no expensive and tricky cathodes or rectifiers are needed, thus making it more versatile. There is an issue with the use of hydrochloric acid as the main acidic source for the stripper in that hydrochloric acid tends to fume when additions or make-ups are performed, as shown in Fig. 18. This is not a pleasant environment for the member of staff that has been tasked with doing this operation.

Figure 18 - Fumes after hydrochloric acid is added to water.
Sulfuric acid-based immersion hard chromium strippers have been developed to reduce the risk of harm arising from this issue of hydrochloric acid fumes. These sulfuric acid-based strippers are also generally faster at stripping than hydrochloric acid strippers, making them more efficient. They attack the base material less aggressively, thus reducing the chances of generating scrap and the resultant waste of resources. They also have a higher loading capability, meaning they last longer before needing replacing again reducing the waste of resources.
Summary
Although the hard chromium process is very mature, there is still much development work being undertaken and there are important advancements in technology being made every year. Although there is no commercial trivalent chromium process available today to replace the existing hexavalent hard chromium plating processes, most of the other hazardous substances commonly being used can be replaced, especially lead and its compounds.
The advancements in systems technology for certain hard chromium plating applications where emissions, exposure and wastage have been reduced are slowly being utilized for the more common plating applications and will lead to safer and more environmentally friendly hard chromium plating.
This means that it is possible to make hard chromium plating greener and there is work being done to make it even greener.
References
1. M.D. Cohen, et al., Crit. Rev. Toxicology, 23 (3), 255 (1993).
2. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 49, Chromium, Nickel and Welding, International Agency for Research on Cancer, Lyon, France (1990); pp. 49-256.
3. EU REACH 348/2013, “Official inclusion of chromium trioxide, chromic acid and chromate substances into Annex XIV of the 1907/2006 REACH regulation,” European Union, (2013).
4. Annette Prüss-Üstün, et al., Comparative Quantification of Health Risks, Chapter 19: “Lead Exposure,” World Health Organisation, Geneva, Switzerland (2004); pp. 1495-1542.
5. Lead, Toxicological Overview, Version 3, UK Health Protection Agency, London, UK (2012).
6. Toxic Chemical Release Inventory Reporting Forms and Instructions, Revised 2012 version, Section 313 of the Emergency Planning and Community Right-to-Know Act, EPA 260-R-13-001, U.S. EPA, Washington, DC (2013); pp. 18-19.
7. F. I. Danilov and A.B. Velichenko, Electrochim. Acta, 38 (2-3), 437 (1993).
8. N. Patton, “Considerations of Pt/Ti to Replace Pb for Chrome Plating Anodes,” Presentation at SUR/FIN 2012, Las Vegas, Nevada, NASF, Washington, DC (2012).
9. Adoption of amendments to Stockholm Convention on Persistent Organic Pollutants, Reference: C.N.524.2009.TREATIES-4 (Depositary Notification), 2009; pp. 18-21.
10. Japan Plating Suppliers Association, PFOS Investigation Committee, “Notice for Discontinuance Production of PFOS Containing Surface Treatment Agents, 26 June 2009.”
11. Environment Canada Response to Comments on the Notice of Intent to recommend export controls for perfluorooctane sulfonate, its salts (PFOS) and lindane; Comment 3; http://www.ec.gc.ca/toxiques-toxics/default.asp?lang=en&n=B978E249-1&xml=B978E249-687D-4278-8719-1B2D1593BE78; Last accessed 15 May 2014.
12. Federal Register/Vol. 77, No. 182/Wednesday, September 19, 2012/Rules and Regulations, Part II, Environmental Protection Agency, 40 CFR Part 63.
13. Neil Patton and Gene Barlowe, “Non-PFOS, Permanent Mist Suppressants for Hard Chrome Plating, Decorative Chrome Plating and Chromic Etch Applications,” Presentation at SUR/FIN 2010, Grand Rapids, Michigan, NASF, Washington, DC (2010).
RELATED CONTENT
-
Expanding on E-Coat
Electrocoat products and processes continue to evolve, with development focused on lower energy use, very low VOC content and methods to reduce cost per applied square foot. The following is a primer on how a combination of new materials and good process control can be used to achieve the highest possible efficiency.
-
Electric vs. Pneumatic Agitators: What Makes Sense?
When determining the need for an agitator, says Mike Elberson from Autoquip, you must look at budget versus long-term energy conservation.
-
Vanadate Conversion Coatings: Alternative to Phosphate?
Emerging pretreatment technology can reduce energy consumption and environmental concerns.



















