The debate of hard gold plating vs. soft gold plating is a common design topic for gold plating applications.
Hard gold plating is a gold electrodeposit that has been alloyed with another element to alter the grain structure of the gold. Soft gold plating is the highest purity gold electrodeposit; it is essentially pure gold without the addition of any alloying elements.
Featured Content
In order to properly specify a gold electrodeposit for an application, five key design considerations should be addressed:
- Wear resistance and contact force. Are there cyclical sliding or switching wear considerations for my application, and what is the design contact force?
- Bonding. Are there sensitive bonding or solderability requirements for my application?
- Corrosion or biocompatibility. What are the corrosion or biocompatibility demands of my application?
- Temperature vs. contact resistance. What is the operational temperature of my application?
- Appearance. Are there any appearance concerns for my application?
Each of these principle design questions are addressed here, specific to the selection of hard gold plating vs. soft gold plating.
Wear Resistance and Contact Force
As state above, hard gold plating is formed when non-noble metallic elements (typically, cobalt, nickel or iron) are alloyed with the gold deposit. These elements alter the grain structure of the deposit, producing a finer grain structure that is more lustrous and more resistant to sliding wear. The grain size of hard gold deposits is about 20-30 µm, whereas soft gold plating has a typical grain size of 1-2 µm. Hard gold commonly has a hardness of 130-200 HK25 (Knoop hardness), while soft gold typically has a hardness of 20-90 HK25 making it very susceptible to sliding wear or wear from contact switching.
The normal force of contact should be considered when specifying hard vs. soft gold plating. Typically, contact force of less than 50 grams is recommended for soft gold applications, whereas hard gold plating performs well under application of 50 grams or more of normal contact force. Due to a lack of insulating oxide or compound formation, soft gold contacts can be used with contact pressures as small as 10 grams and wipe distances as small as 0.010 inch, however, contact force between 15 and 35 grams is recommended.
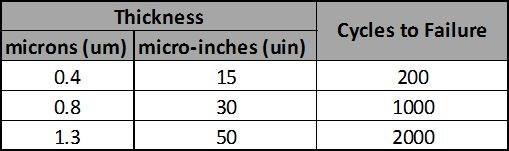
If an application requires repeated sliding wear or make/break switching events, hard gold should be specified. The number of life cycles is proportional to the thickness of the gold; a thicker gold deposit will be able to withstand more cycles. Common thicknesses of functional hard gold deposits range from 0.000015 inch for very low cycle applications to 0.0001 inch for very high cycle applications, with the most common thicknesses ranging between 0.000035 inchand 0.000075 inch.
Table 1 summarizes the results of laboratory testing for the wear-through of a hard gold deposit over a 0.00005-inch nickel underplate. This testing was performed with a 0.250-inch-diameter ball wiped a distance of 0.500 inch under a normal force of 100 grams per cycle.
In addition to the proper selection of hard gold and gold thickness for a gold contact or interconnect, the proper selection of an underplate should be considered to promote gold wear resistance. Common underplates include high-purity sulfamate nickel, bright electrolytic nickel and electroless nickel. A nickel underplate prior to hard or soft gold plating serves many functions, including as a:
- Diffusion barrier. A nickel underplate prior to hard or soft gold plating provides a barrier to solid-state metallic diffusion between copper base metals and alloying metals (such as zinc from brass). This diffusion barrier ensures that weakly bonded intermetallic layers do not form between the base metal and the gold, thereby protecting the integrity of the gold deposit over time. This is especially important for applications at higher temperatures.
- Corrosion inhibitor. A nickel underplate prior to hard or soft gold plating helps promote corrosion resistance. Any pores in the gold layers will lead to the nickel underlay in lieu of the substrate. This generally results in passive oxide formation at the pore base provided the atmosphere does not contain a high degree of acidic corrosives. The nickel underplate prevents the growth of copper oxide or tarnish films to the surface of the gold layer thereby protecting the oxide-free contact surface.
- Leveling layer. A brightened nickel underplate can server to improve the surface finish of the contact surface reducing the coefficient of friction and therefore reducing sliding wear of the gold deposit.
- Load-bearing underlayer. A nickel underplate serves helps bear the contact load of a hard or soft gold plated component. This reduces the potential for cracking of hard gold plated contacts and promotes overall wear resistance.
Bonding Considerations
The presence of “non-noble” elements in hard gold electrodeposits, such as cobalt, nickel and iron, can make soldering more difficult than on soft gold deposits. These co-deposited metals can oxidize at soldering temperatures, reducing the integrity of the solder joint. For very sensitive joining applications such as thermosonic bonding or ultrasonic wire bonding, only soft gold should be considered. In addition, the high purity of Type III soft gold plating is ideal for applications where thermal diffusion of the gold will occur, such as in thermocompression bonding.
The thickness of the gold also should be considered when using tin-lead solder on gold deposits. It has been demonstrated that a tin-lead solder joint that exceeds 3 percent by weight gold will result in embrittlement. For this reason, gold thicknesses of 30 microinches (0.75 mm) or smaller typically are recommended for common tin-lead solder applications.
Finally, beyond the proper selection of hard or soft gold plating for a bonding application, the proper selection of an underplate again should be considered. When soldering to a gold deposit, the initial wetting and solder joint is formed to the gold layer. However, rapid dissolution of the gold into the solder joint results in the final solder bond being made to the underlying layer. Therefore the underlayer must be a robust, solderable surface, or dewetting of the solder joint can occur. A high-purity nickel free of organic codeposits, such as sulfamate nickel, is highly recommended to ensure the most robust solder joint.
Corrosion Resistance and Biocompatibility
As mentioned above, soft gold plating consists of a virtually pure gold deposit free of any intentionally codeposited elements. A properly maintained soft gold plating bath will result in a gold deposit purity of 99.9 percent or more, with total elemental impurity of only 10 ppm or less (typically carbon). By comparison, hard gold plating has intentionally added non-noble elements, and these elements can result in a purity as low as 99.0 percent, with impurities of as much as 2,800 ppm of elements including carbon, hydrogen, oxygen, nitrogen and potassium.
, Since soft gold plating maintains the true noble characteristics of elemental gold, it provides superior corrosion resistance and superior resistance to acid attack or formation of any compounds from elemental exposure. The co-deposited impurities of hard gold reduce the its overall corrosion resistance. In addition, the addition of elements such as cobalt results in oxidation and the formation of other compounds, especially at elevated temperatures.
For these reasons, soft gold plating is superior for medical applications that require biocompatibility. The noble characteristics of a pure soft gold deposit result in a passive coating that will not react with biochemistry within living organisms. In addition, the high purity of soft gold plating can produce a pore-free layer if plated to a sufficient thickness. Finally, the photo opacity of soft gold plating makes it an excellent candidate for devices such as catheters, where visibility of the component during medical procedures is required.
Temperature vs. Contact Resistance
Due to the aforementioned high purity of soft gold plating, soft gold has a lower contact resistance than hard gold plating. ASTM B4882 specifies that the contact resistance of hard gold plating is as much as three times that of soft gold plating, with ohm-per-square readings of 0.10 for hard gold plating vs. 0.03 for soft gold plating.
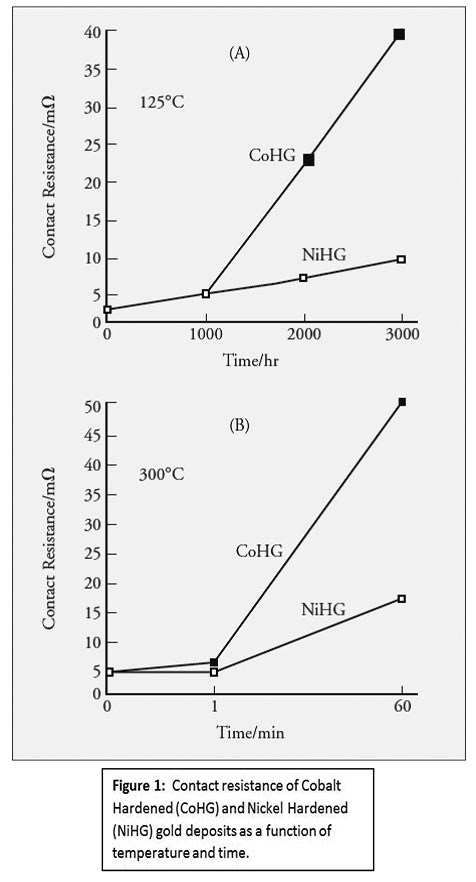
For high-temperature applications, hard gold plating will increase in contact resistance, due to the accelerated formation of oxides and other compounds. A 1998 paper by Yutaka Okinaka and Masao Hoshino cited the increase in contact resistance of both cobalt and nickel hard gold plating as a function of temperature and time. Their research found that significant increases in contact resistance occur after 1,000 hours (41 days) at 125°C. This time frame is reduced to only one minute if the service temperature is increased to 300°C.
Appearance
The smaller and more refined grain structure of hard gold plating results in a finish that is more lustrous or “brighter” than that of soft gold plating. In addition, due to its reduced hardness, soft gold plating is more susceptible to scratching and/or inconsistencies in finish from burnishing or contact with other surfaces. Soft gold plating’s maximum hardness of 90 HK25 is consistent with the hardness of a human fingernail. For these reasons, hard gold plating is generally recommended in applications where a more cosmetically appealing gold contact is required, such as visible interconnect applications.
Once again, in addition to the proper selection of the gold plating, consideration should be given to the underplate. As noted above, an unbrightened sulfamate nickel plating is functionally superior for bonding and soldering applications. However, this unbrightened nickel deposit often results in a more matte gold finish that is less cosmetically pleasing. A bright electrolytic sulfate nickel or bright medium phosphorous electroless underplate deposited prior to hard gold plating will result in a bright gold deposit that consumers commonly associate with quality or superior functionality. It is important to note, however, that although bright gold deposits are more cosmetically pleasing, they often are not functionally superior, especially in bonding or flexing applications. In these cases, a more ductile nickel underplate such as sulfamate nickel and soft gold offer superior bonding and ductility.
Matt Lindstedt is president of Advanced Plating Technologies. Visit advancedplatingtech.com.
RELATED CONTENT
-
Blackening of Ferrous Metals
The reasons for installing an in-house cold blackening system are many and varied.
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.