Is it Time to Revisit your Plastic Metallization Process?
The plating on plastics (POP) market has evolved to include an ever-broader range of substrates, huge increases in selective plating demand, using tools such as two-shot molding and laser ablation.
#research #economics #surfin
Editor’s Note: The following is a synopsis of a presentation given at NASF SUR/FIN 2018, in Cleveland, Ohio on June 4, 2018 in Session 6, Advances in Surface Finishing II. A pdf of this brief can be accessed and printed HERE.
ABSTRACT: There are hundreds of plating lines producing millions of chromium plated parts every day. Almost all are using chemical processes based on chromic acid etching and subsequent catalyzed deposition of electroless nickel or copper, mostly designed decades ago for a different production mix. In the meantime, the plating on plastics (POP) market has evolved to include an ever-broader range of substrates, huge increases in selective plating demand, using tools such as two-shot molding and laser ablation. Understanding the impact of each component of the process and the interaction within the process, allows for a process to be engineered to the demands of modern plastics and the latest designs. Here we will look at critical process steps and function and how they contribute to a robust metallization process for today’s designs.
Featured Content
Introduction
Plating on plastics (POP) is changing. After almost 50 years, the plating on plastic process is changing, driven by many factors. These change factors vary by location, but inevitably affect everyone sooner or later. Such change is a great incentive for suppliers and platers to invest in the next generation of technology. The future of POP will be marked by integrating design and function (e.g., internal LED) and the move to more environmentally friendly products, by the elimination of Cr(VI), fluorinated surfactants, ammonia, formaldehyde, boric acid, phosphates, etc., whilst reducing cost of ownership.
Decorative plating on plastics is an established high-volume process for about 50 years, practiced globally for the supply of chromium finished parts into the automotive, sanitary, fashion ware and household goods markets. The vast majority of plated parts use “full body” plating, where all surfaces are finished with electrolytic chromium.
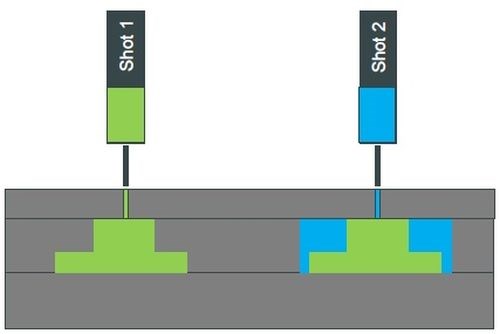
Figure 1 - Two-shot molding.
|
|
|
|
Two-shot, or bimolding, is a technique where two different plastics are injected into the mold, each with different characteristics, which in this case, is that one is plateable and the other not, allowing for selective plating to be delivered without subsequent masking (Fig. 1). The use of two-shot, or multi-molded parts, has increased substantially in the last few years, and shows dramatic future growth, through designs such as LED lighting units for automotive interiors.
Processing these bimolded parts through conventional plating on plastics processes often causes yield loss due to either over-plating on the PC part, or skip plating on the plateable plastic, with continuous problems to achieve the balance between over-plate and under-plate (Fig. 2). Establishing an industrial process window, wide enough to cater for typical variables in base material, molding and design, has been problematic for many platers. Dow has worked to establish a process specifically to manage these yield and process window issues and has high volume manufacturing at global customers using this selective plating on plastic process technology, demonstrating the yield and performance improvements.

Figure 2 - The need for balanced processing in selective POP.
Assessment and optimization
In order to understand and optimize the process for plating on two-shot moldings, and assessment was made of the various stages in the process, including:
- Swell and etch = Structuring
- Catalyst and accelerator = Catalyzation
- Seed layer deposition = Metallization
A scale was created to measure experimental outcomes (Fig. 3). The assessment occurred after electroless nickel. Rank 0 is the aim and preferred, although Rank 1 and 2 can also be acceptable if over-plating on the non-plated PC component is separated from the plated component, as this will dissolve in the acid copper. The plated resin was ABS, while the non-plated resin was polycarbonate (PC).
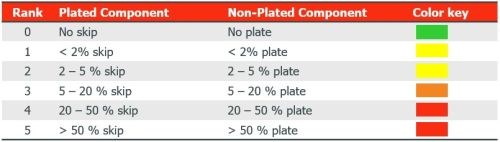
Figure 3 - Assessment scale for evaluation of selective plating skip- or over-plating.
Swell and etch
A variety of customer two-shot samples with ABS (plated) and PC (non-plated) were processed with standard (baseline) etching. The plated ABS component showed an inconsistent surface structure after etch (Fig. 4). At the same time, it is important to avoid attack or structuring of the non-plated PC component. It can be seen that optimizing the structure of the plated plastic, without damaging the surface of the non-plated plastic is an essential component of a robust two-shot plating process.

Figure 4 - Variation in structure of the etched ABS in customer two-shot samples.
During testing it was observed that the chromic acid:sulfuric acid concentration ratio had a significant impact on the structuring of the PC component of the two-shot molding in the etch. Such structuring is undesirable and will lead to selectivity problems.
As with full body plating, the plateable plastic must be prepared for subsequent metallization using optimized swell and etch conditions. These parameters will depend on the plastic type and grade, as well as molding conditions. For ABS, no sweller is typically used, but maintaining a low surface tension is important. For PC-ABS, a sweller is used to increase etch penetration and uniformity. SEM examination (Fig. 5) highlights the contrast between the plateable and non-plateable parts of the molding. This is very important to enable selective catalyzation.
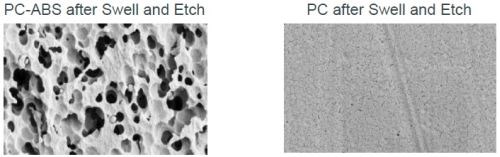
Figure 5 - SEM comparison of the surface morphology of the plateable and non-plateable areas of the two-shot molding
Dow has also established selective process capability with a proprietary chromium-free etchant,** capable of delivering the same capability as traditional chromic acid etching, in terms of selective plating performance. As with Cr(VI) etching, the parameters are dependent on the choice of plastic and the molding parameters, and require the use of a sweller when using PC-ABS materials.
Catalyst and accelerator
Having prepared the two plastics for contrasting structure, the next task is to provide adequate palladium adsorption on the plated plastic, without having palladium remaining on the non-plated plastic. Our design of experiments studies toward process optimization showed that the selection of the appropriate catalyst and accelerator is critical, while optimization of these processes is key (Fig. 6). Every stage in the POP pretreatment is critical to the plating performance.
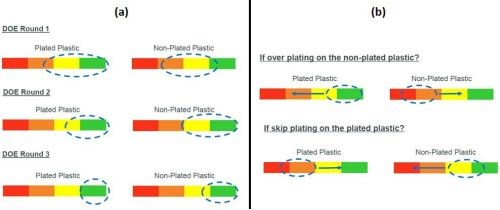
Figure 6 - (a) optimization of catalyst and accelerator; (b) impact of choosing the incorrect product type.
However, no matter which etching or conductive seed layer choice is made, only a very high-quality catalyst, with the correct colloid design, can provide a high yielding, industrial process for bimolded parts. A proprietary Dow catalyst*** provides optimum palladium adsorption on the plateable plastic, without having excess adsorbed palladium on the non-plated plastic. Of course, matching the catalyst with the correct accelerator is essential.† If one chooses the wrong catalyst or accelerator type, achieving selective plating with a robust process window will be very difficult, with significant yield loss. In attempts to adjust a process using the incorrect catalyst or accelerator, the results swing from over plating of the PC to skip plating on the ABS or PC-ABS.
Conductive seed layer
Having optimized the palladium adsorption through the correct choice of catalyst and accelerator, a conductive seed layer can be deposited, allowing subsequent buildup of electrolytic copper, nickel and chromium.
Electroless nickel is preferred in most regions because it has a low operating temperature and is a relatively stable process. However, it usually depends on ammonia for pH control and contains phosphate and salts of boric acid, which are becoming regulated. In addition, the part conductivity may impact the subsequent copper plating and will require an additional immersion or strike copper stage.
Electroless copper is usually more complex to control compared to electroless nickel but provides the benefits of much higher conductivity, no requirement for immersion or strike copper and does not contain phosphate, boric acid or ammonia. In regions where large parts are routinely plated, electroless copper is preferred.
These processes can be operated within their normal stable control window. Many processes need to be forced to operate outside of their design window, to avoid over-plate or skip-plate, by slowing down, or speeding up the electroless baths. Such action inevitably leads to erratic behavior, higher costs, poor control and ironically, no improvement in selective process performance.
Nonetheless, there are environmental concerns. The regulation or focus varies around the globe, but the trend is clear; to minimize the impact on humans and the environment. Electroless nickel contains phosphates, borates and ammonia. Electroless copper contains formaldehyde. Dow has invested in the development of lead-, cadmium- and ammonia-free electroless nickel†† in mass production for years, as well as a formaldehyde-free electroless copper.
Summing up
Having secured selective plating through the correct and optimised choice of products and parameters through etching, catalyzation and seed layer deposition, the buildup of copper, nickel and chromium can be made according to traditional POP plating processes. Depending on the relative size of the plated, versus non-plated areas, care must be taken with racking and anode placements, in order to deliver the best metal distribution. From our studies, the following points are most important:
- Swell and etch - Poor structure, uniformity, depth or contrast will affect selective adsorption of catalyst.
- Catalyzation - The choice of catalyst and pairing with the correct accelerator is critical to achieving selectivity and a wide industrial process window.
- Electroless seed layer metallization - Only a full pretreatment, optimized for selective plating, can allow for stable and robust seed layer metallization.
About the author / presenter

David Wayness is Global Business Development Manager at Dow Chemical Company in Staines, Surrey, United Kingdom.
*Compiled by Dr. James H. Lindsay, Technical Editor - NASF
** Corresponding author:
David Wayness, Global Business Development Manager
Dow Chemical Company
Diamond House Lotus Park, The Causeway,
Staines, Surrey TW18 3AG UK
Phone: +44 7711 976522
Mobile: +44 7711 976522
E-mail: dwayness@dow.com
** Dow Ecoposit™ Chrome Free Etch, Dow Electronic Materials, Dow Chemical Company.
*** Dow Cataposit™ PM-959, Dow Electronic Materials, Dow Chemical Company.
† Dow Accelerator PM-964, Dow Electronic Materials, Dow Chemical Company.
†† Dow Niposit™ PM-988, Dow Electronic Materials, Dow Chemical Company.
RELATED CONTENT
-
An Improvement in the Zincate Method for Plating on Aluminum
Investigations were conducted into the sodium zincate solution used for treating aluminum prior to its being plated. Measurements of the adhesion of the nickel plate to aluminum and its alloys were done to check the effect of modifications made to this solution.
-
White Bronze, Copper-Tin-Zinc Tri-metal: Expanding Applications and New Developments in a Changing Landscape
This paper deals with the renewed interest in applications for white bronze tri-metal (Cu-Sn-Zn alloy).
-
Electroless Nickel-plated Steel versus Stainless Steel: Case Studies
This paper highlights two case studies of manufacturers that have replaced, or done studies to replace, stainless steel with electroless nickel-plated mild steel. In both cases, cost savings could be realized while maintaining or improving product quality.


















