Mechanical Plating
Mechanical plating is an alternative coating system for the impact deposition of zinc and other ductile metals. This article reviews the application technique and performance benefits of this alternative technology to electrodeposition and hot dip galvanizing...
Mechanical plating is an intriguing application technique and has been a commercially accepted process within the North American and European finishing industries for almost half a century. During its history, it has also been referred to as peen plating, impact plating and mechanical galvanizing.
The mechanical plating technique is essentially a batch process for the bulk coating of small parts such as fasteners, washers, springs and spring clips, steel stampings and nails. These products are supplied to diverse markets such as the automotive, aerospace and construction industries.
Featured Content
This technology is an effective means of applying one metal over another as a coated deposit without using electrical current. It is used to apply a number of metallic deposits such as zinc, cadmium, copper, tin, aluminum and other mixed alloys onto a wide range of substrates. Typically deposits are smooth matte to semi-bright in appearance. The brightness can be improved to a degree by a water polish after coating deposition; however, they should be considered functional deposits since the highly leveled and fully bright appearance of an electroplated surface cannot be achieved.
Mechanical deposits can be characterized to some extent by their relative thickness. Standard plating builds are typically 8-12 microns, which compete against coatings applied by electrodeposition. The heavier galvanizing process deposits 40-50 micron coatings and competes against the hot dip galvanizing process.
Processing operates at room temperature using the tumbling action of a barrel to create mechanical energy. In the presence of glass bead impact media and proprietary promoter chemistry, this energy is used to uniformly "cold-weld" powdered metallic particles onto the substrate.
Equipment
Plating Barrel. A plating barrel is the primary piece of process equipment. It is constructed from steel or stainless steel and lined with an inert acid- and abrasion-resistant material such as neoprene, polypropylene or polybutylene. The most effective barrels are of tulip design and have variable-speed capability to generate the tumbling action needed for deposition. Most application takes place at 60 rpm. Correct rotation is important since excessive rotation will produce a lumpy deposit while too slow a speed will not allow the zinc to deposit.
Barrel sizes are typically 0.04-1.13 m3 capacity. The working volume is defined as being 25-35% of the total volume of the barrel.
Separator. This separates the coated parts from the impact media. They can be simple screens with water sprays or more sophisticated mechanical vibratory mechanisms with magnetic separators.
Media Handling. This system returns the impact media from the sump underneath the separator to an overhead storage reservoir to facilitate reuse.
Passivator. This is an off-line arrangement to chromate passivate/top-coat the processed parts. A small series of immersion tanks is generally used.
Dryer. Completed parts, with or without a chromate passivate/top-coat, require drying. Centrifugal (spin) dryers are common and give excellent visual results, but flat belt ovens are used for the larger parts and greater load sizes.
Automation. According to work published by Arnold Satow in Fastener Technology International in 1998, it is possible to offer a degree of automation to the mechanical process that, it is claimed, can offer manpower reductions of 50%. A bar-coding system identifies the work to the process computer, which then calculates and doses the necessary material additions throughout the process. This is yet another example of how technology can reduce the cost base while improving process control capabilities in the finishing industry.
Process Cycle
A typical process cycle for the application of 8-12 microns zinc deposit over a steel substrate:
- Load parts and plating media;
- Add water and rotate barrel;
- Surface preparation (descale/degrease chemistry);
- Copper flash deposition (proprietary chemistry);
- Tin flash coat (promoter chemistry);
- Zinc powder addition (zinc plating);
- Water polish;
- Water rinse; and
- Unload barrel
The total process time is approximately 50 min.
Following unloading, coated parts are separated from the impact media through the separator before transfer to the chromate passivation stage. Further water rinsing and topcoat application (if specified) then follows prior to the drying stage, through spin or horizontal drying methods. For the heavier galvanizing thickness on nails, the passivation stage is generally not used.
Chemistry Additions
Special proprietary chemistry is a vital part of the mechanical plating process. Each process stage occurs in the plating barrel without water rinsing, unlike electrodeposition where water rinsing between each main treatment process is vital to minimize the drag-in of potentially contaminating chemical residues.
Surface preparation, soil and oxide removal are as important in mechanical plating as in any other form of surface coating. A mildly acidic product is used to clean and activate the substrate, followed by the application of a copper strike using a second separate chemical additive to ensure a clean and receptive surface. The third addition is the promoter, helping to generate a controlled deposition rate for bonding the plating metal.
In this way, proprietary products are used to prepare the substrate; a working solution pH of 1 to 2 in the process barrel is needed to promote adhesion and high deposition efficiency. This pH range maintains an oxide-free condition of the substrate and the metallic plating particles, enabling effective mechanical bonding. Additions of proprietary chemistry are made based upon the total surface area of parts to be processed; therefore, it is important for the applicator to calculate this for each type of product to be processed. Chemical additions will be expressed in g/m2 for powdered products and ml/m2 for liquids. The material product data sheet from the chemical supplier will give specific guidance for the correct addition quantities. Since each chemical product is calculated for use on each individual load, it will be consumed and must be added for each separate barrel load.
Impact Media
The impact media beads are clear, colorless glass spheres made of soda lime glass. This material is produced from silicon dioxide (sand), calcium carbonate (limestone) and sodium carbonate (soda ash). Chemically inert and non-toxic, with a low coefficient of friction and high wear resistance, they can be recycled and reused many times, ensuring their cost effectiveness.
The media serve several functions in the mechanical plating process: they assist surface preparation through a mild abrasive action; promote the dispersion of chemistry within the barrel; help separate parts; and provide energy transfer for even metallic deposition across the substrate surface.
A general rule of thumb is to add a 1:1 mix of glass beads and parts to the barrel for plating, while a 2:1 mix is recommended for galvanizing. The media are available in a variety of sizes, expressed in mm or mesh ratings. A common mix uses several distinct sizes in the process batch, some larger at 4-5mm with the smallest known as mush beads. A typical mix would be:
| 50% v/v | 4-5 mm |
| 25% v/v | 2-2.5 mm |
| 25% v/v | 1-1.25 mm |
Three-quarter to one-mm beads (mush) can be added to an initial mix, but the smaller beads soon reduce in size naturally. It is important to check that the media does not lodge into areas of the parts; typically this would be recessed heads on fasteners.
Plating Material
The plating metal is supplied in the form of a dry, fine, metallic powder, which is added to the barrel load based upon total surface area and the deposit thickness required. Larger installations processing loads of 1,000 kg tend to premix the powder into a liquid-based water slurry for easier addition and effective dispersion within the barrel load.
Additions will be expressed as gm/m2 per micron. When making dry powder additions, to ensure even coating distribution throughout the entire load and to achieve optimum thickness uniformity, it is advisable to split the addition of metallic powder into a number of smaller ones. Three to four would be used to achieve a deposit of 8-12 micron thickness, while 6 and above is advised for the larger galvanizing deposits.
Corrosion Protection
The application of zinc-based coatings onto ferrous substrates for the benefit of improved corrosion protection is well established. Zinc offers sacrificial protection because it has an anodic corrosion potential to steel; therefore, it will corrode preferentially. Chromate passivation coatings extend time to the formation of oxide products, commonly known as white corrosion.
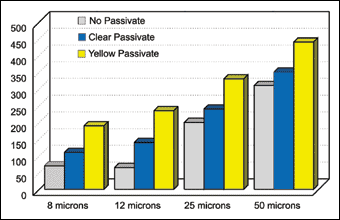
Mechanically applied zinc deposits offer good corrosion protection, Figure 1. Their performance is comparable to those applied using electrodeposition. The corrosion performance of coating systems are often measured by the 5% neutral salt spray test, ASTM B117, which continues to be the most accepted and recognized test method for performance testing.
Technology Advantages
Mechanical processing is versatile because deposits can be readily applied onto a diverse range of substrates, including ferrous metals, copper alloys and stainless steels; of particular note is its ability to process diecastings and powder-sintered materials.
A significant reason for its use is the avoidance of hydrogen embrittlement, since the process itself will not cause this phenomenon. This ensures that in many applications it has become the preferred coating method for hardened fasteners and stressed components. This also eliminates the need for the costly pre-plate and post-plate baking operations as associated with electrodeposition high-strength steels. Since the mechanical process operates at room temperature, it will not detemper heat-treated parts.
Mechanically applied deposits are extremely uniform in nature, often being able to coat surface areas that prove difficult with electrodeposition. Mechanical coating thickness distribution is generally better than comparable electroplated and galvanized coatings. This means that threaded parts do not require resizing after processing. The process can plate up to 75 µm and still maintain a uniform deposit. It also has the ability to process flat parts, such as washers, without masking.
Mechanical processing uses relatively simple, non-cyanide, non-toxic chemistry that is consumed during each process cycle. Modern proprietary chemistry does not use complexing agents. The high process efficiency of more than 90% means that only small zinc levels are present in wastewater, thereby treatment is easy and waste treatment costs are reduced. A simple pH correction and dose of polyelectrolyte for effective flocculation is generally all that is required.
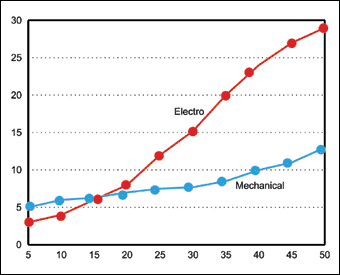
The mechanical process is cost effective, requiring comparatively little energy. Unlike electrodeposition, it does not require any special jigging or anode configuration. Its economics is especially attractive for deposits above 10-15 microns. This is because only slightly longer addition times are needed to achieve greater thickness, unlike electrodeposition where plating time is directly proportional to thickness. Figure 2 offers a greater cost analysis for this comparison.
Mechanical plating offers a credible and cost-effective coating alternative to electrodeposition and hot dip galvanizing technologies for the manufacturers of fasteners, nails, washers, springs and steel stampings. Mechanically applied deposits provide a uniform coating thickness, and offer excellent corrosion protection while avoiding the problems of hydrogen embrittlement.
To learn more visit Atotech North America.
RELATED CONTENT
-
Sizing Heating and Cooling Coils
Why is it important for you to know this?
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.