Mechanical Properties of Electroformed Metals
In 1996, the AESF held its highly regarded electroforming course, prepared by Ron Parkinson for presentation by The Nickel Development Institute (NiDI) and the AESF. What follows is a slightly modified excerpt, specifically on the mechanical properties of electroformed metals. Much of this information has withstood the test of time, and gives a perspective of this technology at the turn-of-the-century.
#nasf
An Excerpt from the AESF Electroforming Course - 1996
Editor’s Note: In 1996, the AESF held its highly regarded electroforming course, prepared by Ron Parkinson for presentation by The Nickel Development Institute (NiDI) and The American Electroplaters and Surface Finishers Society (AESF). What follows is a slightly modified excerpt, specifically on the mechanical properties of electroformed metals. Much of this information has withstood the test of time, and gives a perspective of this technology at the turn-of-the-century A printable PDF version of this article is available by clicking HERE.
Featured Content
Introduction
The mechanical properties of electrodeposits have been studied in great detail. Of the various metals used in electroforming, nickel has been studied most extensively and with good reason. It is normally the metal of choice for structural, electroformed components as it can provide high strength, and good ranges of ductility, hardness and internal stress. It is important for designers and engineers to be aware of these properties and for electroformers to know how to control them. The mechanical properties of copper are of much less importance as copper is often selected for other reasons. These may include cost, deposition rates or thermal and electrical conductivity properties. In this article, the mechanical properties of electroforms and methods used to control them are reviewed.
Mechanical properties of nickel
A major advantage of nickel for electroforming is the range of properties that can be obtained. In this section, the properties of deposits from Watts-type and sulfamate electrolytes are described. Information on the effect of electrolyte composition, operating conditions, impurities and additives is provided and emphasis is placed on sulfamate solutions - the most important to the electroforming industry. It should be pointed out that, with an understanding of the effects of variables in the electroforming process and the establishment of routine maintenance practices, these electrolytes have the ability to provide consistent results indefinitely.
Watts-type solution
There are a very limited number of applications for this electrolyte and the large volume of Watts-type solutions now in use can be attributed almost entirely to the rotary printing screen industry. In this industry, internal stress is the property of most interest as its control in the compressive state is required to enable electroforms, sometimes several meters long, to be removed from the cylindrical mandrels. This is not intended to suggest that other properties such as tensile strength and ductility are of no concern but under well-established operating conditions within normal limits, they should show little variation.
The effect of electrolyte temperature, pH and cathode current density on hardness, elongation and tensile strength have been well documented. Results are shown in Figures 1, 2 and 3 and it can be seen that under the typical operating conditions shown in Table 1, there are no dramatic changes in these properties. Outside typical operating ranges, it is interesting to see that very low current densities and especially very high pH values have significant effects on deposit properties and are usually considered to be detrimental.
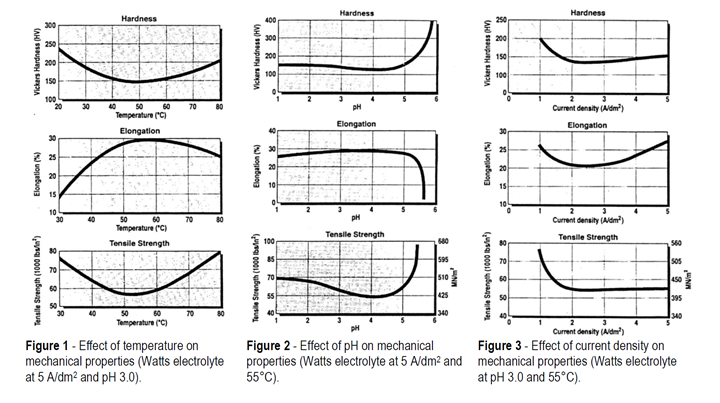
Table 1 - Composition and operating conditions of Watts-type solutions
Nickel sulfate (NiSO4•6H2O) 240 – 300 g/L
Nickel chloride (NiCl2•6H2O) 35 – 55 g/L
Boric acid (H3BO3) 30 – 45 g/L
Temperature 40 – 60°C
pH 3.5 – 4.5
Cathode current density 3 – 10 A/dm2
Deposition rate 36 – 120 μm/hr
Agitation Air or mechanical
Within the normal operating range for Watts-type electrolytes, the internal stress is typically 125 to 185 MN/ m2 or 18,000 to 27,000 psi tensile. These are high values compared with stresses obtained in deposits from typical sulfamate electrolytes and are not generally acceptable for electroforming. Within the normal operating ranges, the effects of electrolyte temperature, pH and cathode current density would not have a great effect on internal stress, although undoubtedly higher current densities tend to increase tensile stress. Most frequently, therefore, Watts-type solutions are used with the addition of a stress reducer.
Stress reducers used in nickel electroforming are certain sulfur-bearing organic compounds. They can be very effective at low concentrations, and by careful monitoring of their rates of addition and consumption, it is possible to control stress at a selected level, tensile or compressive. These additives vary in their effectiveness and they are not all capable of producing a compressive stress at acceptable concentrations, as they have a very significant effect on other properties. For instance, they increase tensile strength and hardness and reduce ductility. They also co-deposit sulfur with the nickel, which has a most deleterious effect if the electroform is to be used at elevated temperatures. This is reviewed in some detail later when the properties of deposits from sulfamate solutions are described. Typical stress reducers in order of their effectiveness are saccharin, paratoluene sulfonamide, benzene disulfonate and 1-3-6 sodium naphthalene trisulfonate. The effect of these additives on deposit stress from Watts-type electrolytes is shown in Fig. 4.
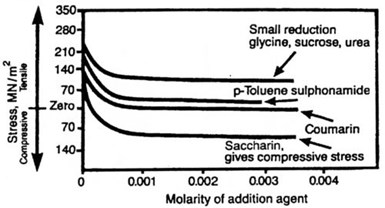
Fig. 4 Effect of organic additives to Watts electrolytes on internal stress.
The limited range of properties available from Watts-type electrolytes, the high internal stress, the incorporation of sulfur if tensile stress is to be reduced and the often deleterious effect of this sulfur are generally regarded as negative aspects. However, the dominance of Watts-type electrolytes in probably the largest single application for nickel in electroforming, e.g. rotary printing screen production, is evidence of the high performance attainable by strict control of operating conditions in this high-quality industry.
Other parts of the industry are almost entirely dependent on the use of nickel sulfamate electrolytes and with good reason. They have more to offer the electroformer than any other electrolyte used throughout the industry.
Nickel sulfamate solutions
The importance of nickel sulfamate electrolytes to the electroforming industry is enormous. The number of applications in which they are used greatly outweighs all others. They are simple to control and a wide range of properties can be obtained by adjustments to their composition, the operating conditions and by the use of various additives. In recent years, much work has been done to expand the applications for nickel electroforming and this has resulted in a growing interest in nickel alloy deposition, especially nickel-cobalt. In this section, the effect of composition, operating conditions and solution additives on deposit properties are described for nickel sulfamate solutions.
In Table 2, the typical properties of nickel deposits from Watts-type, sulfamate and fluoborate electrolytes are compared. It can be seen that sulfamate deposits have typically higher tensile strength, greater hardness and very much lower tensile stress than Watts-type deposits. Elongation is very similar. The range of properties shown satisfy most electroforming requirements, but it is essential that designers and engineers are aware of the extended range that can be obtained. Obviously, it is equally important that electroformers are aware of how this range can be extended and properties controlled by process modifications and the use of additives.
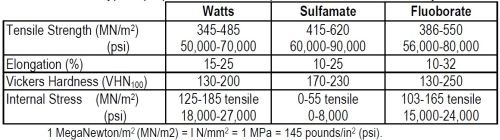
Typical properties of nickel deposited from various electrolytes.
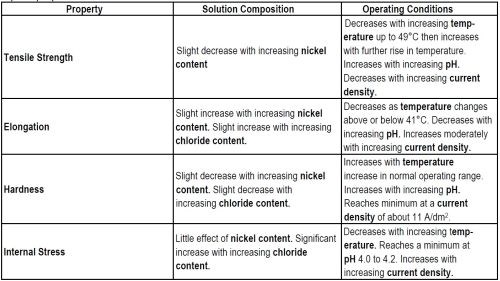
Figure 5 shows the qualitative effects of operating conditions on the properties of nickel sulfamate electrodeposits The most important trends for electroformers to learn from these graphs are shown in Table 3.
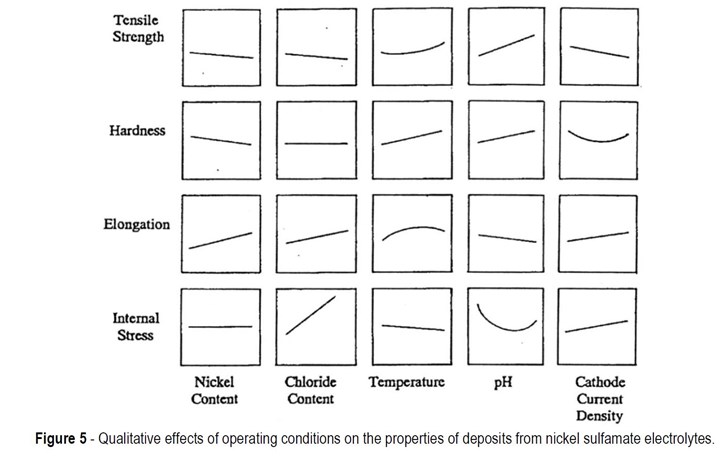
Table 3 - Trends relating solution composition and operating variables in nickel sulfamate solutions to deposit properties.
Inorganic Impurities
Most inorganic impurities are metallic. Others of concern in sulfamate electrolytes are ammonium and sulphate ions. The greater sensitivity of sulfamate solutions than Watts solutions to metallic impurities is evident from the recommended allowable maximum concentrations shown in Table 4. The general effects of these impurities are also shown, together with the typical concentrations found in nickel sulfamate electrolytes.
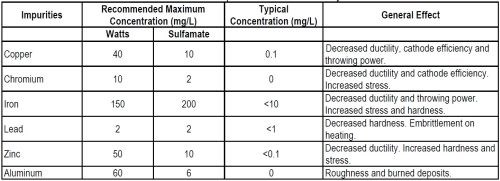
Table 4 - Metallic impurities in nickel electrolytes.
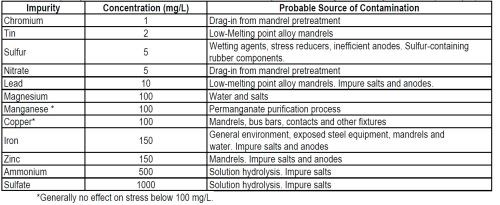
Although it is important to maintain the concentration of impurities in Watts solutions at low levels, it is less important than in sulfamate because it is normal practice when using Watts solutions in electroforming to control stress by adding organic stress reducers. These may have a greater effect than the impurities on other properties such as hardness and ductility. However, one of the major advantages of nickel sulfamate solutions is the low internal stress attainable without the use of additives, but to maintain this, the buildup of impurities must be avoided. Zero or very low tensile stress is preferred in most electroforms and the effect of the impurities on stress has been studied extensively. The concentration of impurities that will cause an increase in internal stress by 7 MN/m2 (1000 psi) and the most probable sources of these impurities are shown in Table 5.
The methods used to remove the typical organic impurities normally associated with electrodeposition, e.g. oils, grease and general contaminants continuous filtration through activated carbon, and batch treatment in activated carbon in conjunction with hydrogen peroxide or potassium permanganate. Good maintenance practices and continuous carbon treatment are usually used in electroforming operations to eliminate such impurities and their adverse effect on deposit properties, such as pitting.
When using nickel sulfamate electrolytes, there is another potential source of organic contamination that can have a disastrous effect on deposit properties if not recognized. The source is the sulfamate ion itself and the organic contamination is a compound produced by oxidation of this ion at the surface of an unsatisfactory anode. The choice and performance of anode products are therefore of extreme importance in electroforming from nickel sulfamate solutions.
In most nickel electrodeposition processes, nickel anodes normally operate at 100% efficiency, or very close to that value. This is due to the fact that most electrolytes contain sufficient chloride to provide satisfactory anode dissolution. It is considered by many electroformers to be good practice to maintain some chloride in sulfamate electrolytes to ensure satisfactory anode dissolution rates and thereby prevent the formation of undesirable organic compounds by oxidation of the sulfamate ion at the anode surface. If little or no chloride is used, the anode of choice must be the sulfur depolarized type. However, although chloride will increase tensile stress, many electroformers operate with chloride concentrations that are sufficiently high to allow them the choice of sulfur depolarized or high purity anodes. In selecting an anode therefore, consideration must be given to acceptable chloride contents, deposit stress levels, current densities and the use of stress reducing additives, but the primary requirement is to ensure satisfactory anode dissolution.
Evidence of poor anode performance will quickly become evident. The pH of the electrolyte will decrease, and the appearance of the deposit will change from dull, matte grey to bright. This will be accompanied by a decrease in tensile stress and eventually a change from tensile to compressive stress. The deposit will become brittle and some brown discoloration may occur at the anode surface. These changes occur because, at an inefficient anode, a compound, identified as azodisulfonate, will be produced by oxidation of the sulfamate ion. This is a sulfur-bearing organic compound that is readily codeposited with the nickel at the cathode. It can have a disastrous effect on electroforms but the problem can be avoided by selecting an appropriate anode and operating conditions.
Under well-controlled conditions, it is possible to take advantage of this unusual reaction at an inefficient anode. It can be used to control stress if an insoluble anode is used in conjunction with a satisfactory anode. For instance, the amount of the stress-reducing organic compound produced can be controlled by passing a small, known percentage of the total current through the insoluble anode, with the balance being carried by the regular, efficient anode. Typically, the insoluble anode would be installed in a small auxiliary tank through which the bulk electrolyte is continuously recirculated, and a separate rectifier would be used to provide, say, 1 to 5% of the total current.
Practical methods used to control nickel properties
The versatility of nickel sulfamate electrolytes and the range of deposit properties that can be obtained have been responsible for its dominant position in the electroforming industry. As described earlier, a very useful and acceptable range of properties can be obtained simply by controlling operating conditions, but there is now considerable demand for properties outside of this range. For instance, higher hardness values are often required, and in such cases, additives to the electrolyte can be used. However, additives can affect several properties and so there is often a need to compromise. As an example, the use of organic additives to increase hardness can have an adverse effect on the elevated temperature properties of nickel electroforms and so may not be acceptable. Practically all additives will have some effect on one of the most important properties of the deposits, i.e., internal stress. Electroformers must be aware of the relationships between additives and deposit properties and these are described below for the additives most frequently used.
The properties of most interest to nickel electroformers are usually internal stress and hardness. Occasionally, there are specific requirements for tensile strength and ductility but these are less common. Such requirements may exist in structural components that are used in the aerospace industry, for instance.
Effect of additives on internal stress and hardness
(a) Chloride and bromide
Chloride is not an absolute essential constituent of a nickel sulfamate electrolyte and so it can be considered as an optional additive. However, in view of its beneficial effect on anode efficiency and the importance of this in preventing the formation of organic stress reducing compounds, some chloride is frequently used.
Chloride will increase tensile stress and so the optimum concentration is dependent on the acceptable stress levels. Consideration should also be given to operating conditions and their effect on anode efficiency, e.g., current density, when selecting a chloride concentration. Chloride is usually added as nickel chloride.
Nickel bromide is sometimes used as an alternative to nickel chloride, but
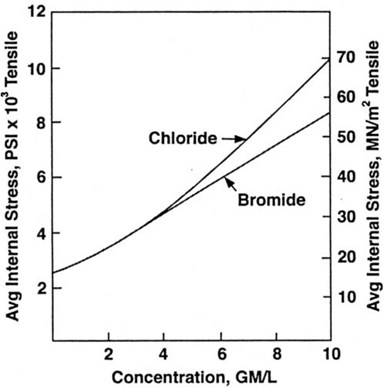
Figure 6 - Effect of chloride and bromide on internal
stress of nickel deposits from sulfamate electrolytes.
this is rare. The only possible advantage is that it is reported to have less effect than chloride on stress at high concentrations. However, at high anode potentials, bromide can rapidly destroy titanium anode baskets.
The effect of chloride and bromide ion on stress is shown in Fig. 6. Operating conditions were pH 4.0, temperature 49°C and current density 2.7 A/dm2. The increase in tensile stress with increased halogen ion content is evident. It can be seen for instance, that the addition of 3 g/L chloride, (equivalent to 10 g/L nickel chloride hexahydrate) to the sulfamate electrolyte increases stress from about 18 MN/m2 (2500 psi) to about 28 MN/m2 (4000 psi). This would be an acceptable level for many electroforms. Very few electroforming operations would operate at chloride concentrations as high as 6 g/L and at this level, bromide is shown to have no significant advantage over chloride. Also, at these concentrations, chloride and bromide have been reported to have no significant effect on the hardness of nickel deposits.
(b) Organic additives
Organic addition agents are frequently used to control stress or hardness. They have a very significant effect on both these properties. Hardness is increased and tensile stress is reduced to the point where it becomes compressive as the concentration of additive is increased. Therefore, by control of the rate of addition, these additives can be used to control the internal stress in the low tensile state, at zero or in the compressive state.
Common stress reducers used in nickel sulfamate solutions include benzene sulfonic acids and their derivatives, naphthalene sulfonic acids and their derivatives, para-toluene sulfonamide and saccharin. The latter is by far the most commonly used. These are all sulfur-bearing organic compounds, which cause sulfur to be codeposited with the nickel, thereby greatly reducing the grain size of the nickel deposit. This in turn, significantly moves the stress in the compressive direction and increases the hardness and the brightness of the nickel.
The fact that nickel electroformed from solutions containing these organic additives contain sulfur, limits the use of the electroforms to low temperature applications. The maximum acceptable temperature depends on the sulfur content of the nickel and the length of time exposed to the elevated temperature, but it is generally recommended that temperatures do not exceed 200°C. The reason for this is that sulfur seriously embrittles nickel exposed to temperatures at which some change in metallurgical structure can occur. For instance, when nickel is fully recrystallized, almost all the sulfur present will migrate to the grain boundaries and form an intergranular network of nickel sulfide. This has drastic consequences for all physical properties such as ductility and tensile strength. Codeposition of manganese can alleviate this problem.
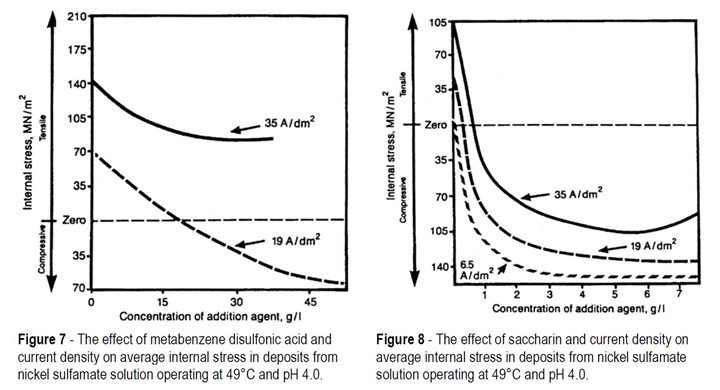
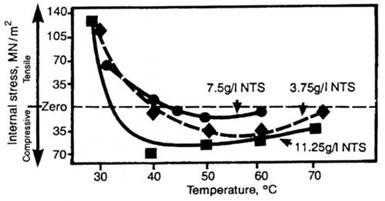
Figure 9 - The effect of 1:3:6 naphthalene trisulfonic acid and solution temperature on average internal stress in deposits from nickel sulfamate solution.
However, these sulfur-bearing organic additives are widely used to control stress and hardness of electroforms which will not be used at elevated temperatures. The effects of some of these additives on stress are shown in Figs. 7, 8 and 9.
Figure 7 shows two plots of deposit stress versus concentration of addition agents for a 327 g/L nickel sulfamate solution containing metabenzene disulfonic acid, operated at pH 4.0 and 49°C. The results show that the general effect of the addition agent is to cause stress to move in the compressive direction. For a given concentration of addition agent, the stress is more compressive or less tensile at the lower of the two current densities used.
Saccharin is a more powerful stress reducer than metabenzene disulfonic acid, as is shown by the three stress curves in Figure 8 that are for the same 327 g/L solution used under the same plating conditions. The addition agent reduces tensile stress and, at sufficiently high concentration, makes it compressive. There is a limit to the amount of compressive stress that can be produced, and when that stress is reached, further additions of saccharin have no effect on stress. Just as with metabenzene disulfonic acid, in a solution containing a given concentration of saccharin, lowering the current density causes stress to move in the compressive direction. This parallels the effect of current density in solutions that do not contain an addition of stress-reducer.
A practical point to note when trying to achieve zero stress with saccharin in sulfamate solution is the steepness of the curves at the zero-stress level. This means that small changes in concentration of saccharin have a large effect on stress and so maintaining zero stress requires great care. Automatic addition of dilute saccharin solution based on predetermined consumption rates should be used.
The effect of solution temperature on deposit stress is shown in Figure 9 for a solution containing 300 g/L of nickel sulfamate plus 3.75, 7.5 or 11.25 g/L of naphthalene trisulfonic acid in the form of its sodium salt. The results show that, as temperature is increased, the stress moves in the compressive direction, rapidly between 30 and 40°C and then more slowly. Above 40°C, the effect levels off. The initial move in the compressive direction again parallels what happens in solutions free from organic stress-reducing additives.
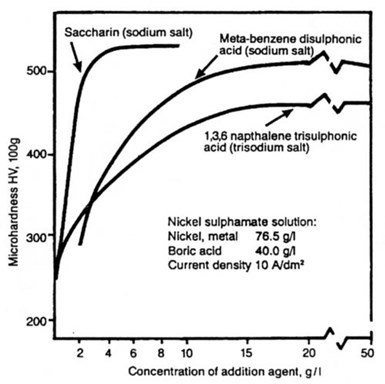
Figure 10 - The effect of organic additives on the hardness of nickel deposited from sulfamate solution.
The effects of these same organic additives on the hardness of nickel deposits are shown in Figures 10, 11 and 12.
Figure 10 shows the relationship between concentration of addition agent and deposit hardness for a conventional nickel sulfamate solution containing naphthalene trisulfonic acid sodium salt, metabenzene disulfonic acid sodium salt, and saccharin, as the sodium salt. The effect of all three compounds is similar. Deposit hardness increases rapidly with increasing concentration of addition agent, until, at a certain concentration for each compound, further additions have little effect. The highest hardness is obtained with saccharin and, as in the case of its function as a stress-reducer, this compound is the most potent of the three additives.
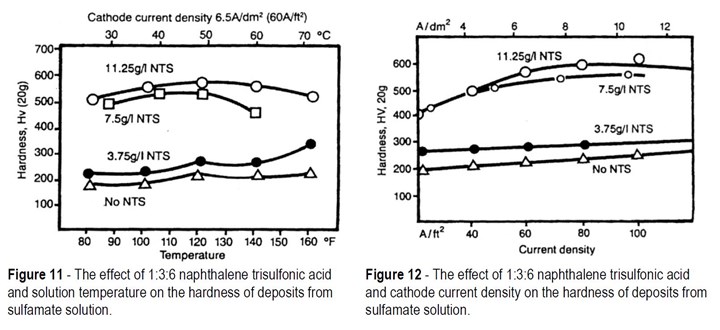
Figure 11 shows the relationship between solution temperature and hardness for deposits from sulfamate solutions containing 0, 3.75, 7.5 and 11.25 g/L naphthalene trisulfonic acid. The effect of solution temperature over a wide range is of little significance compared to the effect of additive concentration.
Similarly, for the same solutions, Fig. 12 shows that current density also has much less effect on hardness than additive concentration.
In summary, therefore, organic additives can be used in sulfamate solutions to shift internal stress of nickel electroforms in the compressive direction and to increase hardness. The changes will be accompanied by a decrease in ductility and the codeposition of some sulfur. The presence of sulfur limits the electroforms to applications in which they will not be exposed to temperatures exceeding about 200°C. Severe embrittlement can occur at higher temperatures, although there is a method of preventing this, by manganese additives.
Organic additives are also used to suppress the growth of nodules and improve current distribution. Such additives are known as leveling agents. In electroforming, it is important to prevent the initiation of nodule growth as much as possible because once formed, their growth is self-perpetuating and during the long deposition times often required, they may grow to an unacceptable size, which seriously effects overall current distribution.
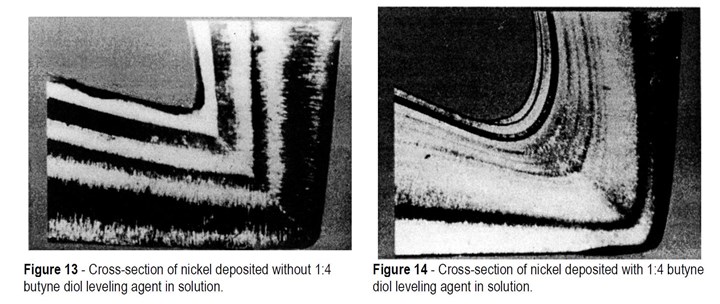
Leveling agents are also used beneficially to produce radiused deposits in sharp corners. This prevents the formation of boundary planes as shown in Fig. 13 which have been described as planes of weakness formed between the two growing faces of the deposit. There is not complete agreement that boundary planes are indeed planes of weakness, but nevertheless poor current distribution in internal corners is generally recognized as a problem. The corners are low current density areas which will result in thinner than average deposit thickness and the preferential codeposition of any metallic impurities that may be present in the electrolyte. Both can result in areas of weakness. In addition, sharp corners can provide notch areas that could result in fatigue failure if the electroform is required to operate under fluctuating stress conditions. The advantages obtained in using 1:4 butyne diol to improve deposit quality in sharp corners is demonstrated in Fig. 14.
Organic leveling agents are also used to great advantage in the electroforming of certain screen products. In the production of high precision printing screens, for instance, it is often required to control the lateral growth of the nickel deposit so that hole size can be controlled as the deposit thickness increases. Ethylenecyanohydrin and hydroxy-propionitrile have been reported as suitable leveling agents for this application.
In general, leveling agents tend to increase the internal stress of nickel electroforms in the tensile direction. The breakdown products from organic additives generally have the same effect. However, a great advantage in using 1:4 butyne diol is that the breakdown products that it generates can be easily removed by continuous filtration through activated carbon, without removing the additive itself. It is therefore possible to control stress very closely.
(c) Nickel/cobalt alloys
Cobalt has a marked effect on the properties of electrodeposited nickel. It increases tensile strength and internal stress, reduces ductility and most importantly for electroformers, it increases hardness. The hardness of nickel/cobalt alloys varies with their cobalt content and in electroforming from sulfamate solutions, cobalt content is dependent on several factors. The dominant factor is the ratio of nickel to cobalt concentrations in the electrolyte, but cathode current density and the degree of solution agitation also have an effect. The importance of cobalt for increasing the hardness of nickel electroforms is that unlike deposits produced using organic additives, the nickel/cobalt alloys can be used at elevated temperatures without suffering embrittlement.
Nickel/cobalt alloys can be produced from conventional nickel sulfamate electrolytes simply by adding cobalt sulfamate. As usual, nickel anodes are used to replenish the nickel plated out of solution and cobalt sulfamate is added at a controlled rate to replenish the cobalt and maintain the required nickel/cobalt ratio in solution.
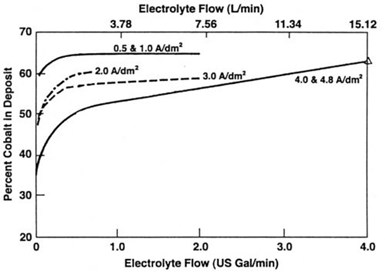
Figure 15 - Relationship between cobalt content of deposit, current density and agitation in a nickel/cobalt sulfamate electrolyte with a Ni/Co ratio of 10:1.
When electroforming nickel/cobalt alloys to meet specific property requirements such as hardness, it is important for electroformers to determine the optimum operating conditions for their own installation. This generally involves establishing the required nickel/cobalt ratio in solution using proposed operating conditions, with major consideration being given to current density and solution agitation. As an example of the importance of these factors, it has been reported that at a nickel/cobalt ratio in solution of 10:1 and a current density of 2.7 A/dm2 (25 A/ft2), the cobalt content of the deposit can change from 28.5% with no agitation to 50% with moderate agitation and 53.5% with vigorous agitation. It has further been reported that the influence of current density and agitation on the composition of the deposit is due to concentration polarization of the Co2+ ions. This is supported by Fig. 15. It shows that at lower current densities, it requires little solution agitation to obtain stable cobalt content in the deposit but at higher current densities, at which concentration polarization will occur, stability is more difficult to attain even with increased agitation.
The effect of current density and the nickel/cobalt ratio in solution on cobalt content in the deposit is shown in Fig. 16. It can be seen that at all nickel/cobalt ratios, a decrease in current density results in an increase in the cobalt content of the deposit.
A typical graph showing the relationship between nickel/cobalt ratio and cobalt content of the deposit when using a current density of 3 A/dm2 is shown in Fig. 17. It is of interest, not only because of the actual cobalt contents shown but because of the very steep slope of the curve, which clearly indicates that strict control of electrolyte conditions must be maintained, especially for alloys containing up to about 50% cobalt.
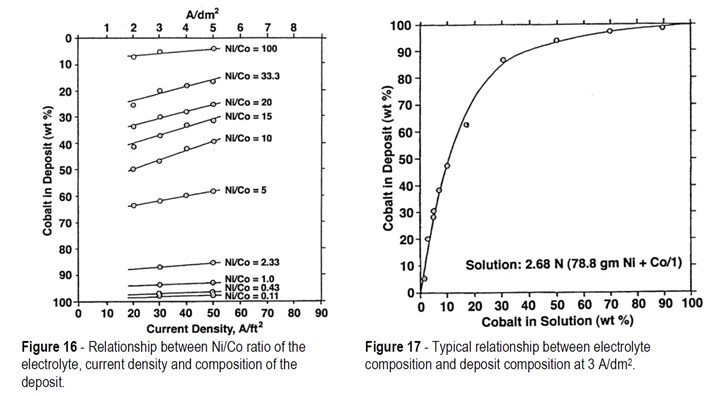
From this figure, it can be seen that it requires the cobalt concentration in the electrolyte to be only about 10% of the nickel concentration to obtain alloy deposits containing 50% cobalt. Rarely would electroformers be required to produce nickel alloys with greater cobalt content and so the relationship between electrolyte cobalt concentrations up to 10% of the nickel concentration and physical properties of the deposit are of most importance. In Fig. 18, the effect of cobalt concentration on the hardness, ductility and internal stress of the alloys is shown. Although a great increase in hardness can be achieved, it is accompanied by a significant increase in internal stress and a decrease in ductility.
Although Fig. 18 shows that hardness values up to 500 VHN are possible at high cobalt levels, the increase is accompanied by a significant change in the internal stress in the tensile direction. This is not always acceptable, as it could result in deformation of the electroforms, but stress can be reduced to acceptable levels by controlled additions of organic stress reducers, such as saccharin. However, the codeposition of sulfur from organic stress reducers limits the use of the electroforms to low temperature applications, i.e., not exceeding about 200°C, as described earlier. If it is necessary to use stress reducers, it is still possible to produce nickel/cobalt alloys that can be used at elevated temperatures if a further modification to the electrolyte is made. This requires the addition of small concentrations of manganese to the electrolyte.
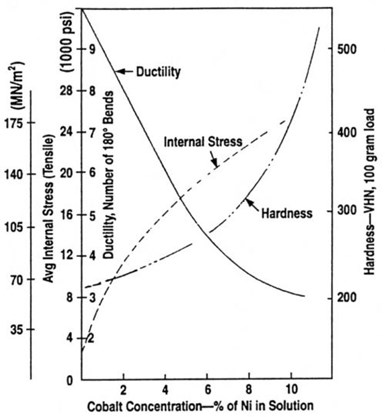
Figure 18 - Relationship between electrolyte cobalt content and physical properties of deposits.
Nickel/cobalt alloys are also useful when very high strength is required in electroforms. For instance, it has been reported that ultimate strengths exceeding 1520 MN/m2 (220,000 psi) and yield strengths exceeding 1100 MN/m2 (160,000 psi) are possible.
(d) Nickel/manganese alloys
It is well known that the mechanical properties of nickel are drastically effected by heat treatment in the presence of sulfur. For instance, electrodeposited nickel containing sulfur can become seriously embrittled on exposure to temperatures above about 200°C due to the formation of brittle nickel sulfide in grain boundaries as the recrystallization process occurs. The recrystallization temperature will vary with the purity and metallurgical condition of the deposit and 200°C is a very safe value. In fact, recrystallization below 400°C is rare.
In nickel electroforming, sulfur-bearing organic compounds are frequently used to control internal stress and they cause sulfur to be codeposited with the nickel. Such electroforms are therefore not normally used at elevated temperatures but a method of overcoming this problem has been developed. All forms of nickel are susceptible to sulfur attack and yet in the metallurgical industry, certain grades of nickel can be used without embrittlement at elevated temperatures even though they contain sulfur as an impurity. This results from the addition of manganese or magnesium to the molten nickel immediately prior to casting. Both these elements have the capability of combining with the sulfur preferentially and preventing the formation of nickel sulfide in grain boundaries. Similarly, codeposition of manganese during nickel electroforming will prevent the drastic effect that sulfur from organic additives can have on mechanical properties at elevated temperatures.
Manganese does not co-deposit with nickel as readily as cobalt, but nevertheless it can be done very simply in either Watts or sulfamate electrolytes. In Watts electrolytes, manganese can be added as manganese sulfate or in sulfamate electrolytes as manganese sulfamate. The amount of manganese required to prevent high temperature embrittlement due to sulfur is obviously dependent on the sulfur content, but also on the operating temperature of the part. It is therefore very important when using sulfur-bearing additives for reducing tensile stress and manganese for overcoming the adverse effect of sulfur at elevated temperatures that the sulfur content of the nickel be strictly controlled. Only then can the required concentration of manganese be determined and codeposited. It is generally understood that a manganese to sulfur ratio of up to 5:1 may be required but this should be determined under the operating conditions of the electroform.
Manganese content of the deposits increase as current density is raised and typical manganese concentrations in the electrolyte are 5 to 10 g/L. Manganese increases tensile stress and hardness of the deposits.
Mechanical properties of copper
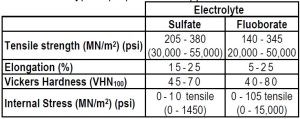
Table 6 - Typical properties of copper electroforms
Typical properties of copper deposits obtained from the sulfate and fluoborate electrolytes are compared below in Table 6.
As with nickel, these mechanical properties are influenced by changes in electrolyte composition and operating conditions, but more dramatically with the use of organic additives. The influence of operating conditions and electrolyte composition on the mechanical properties of deposits from the sulfate and fluoborate electrolytes are given in Tables 7 and 8, respectively.
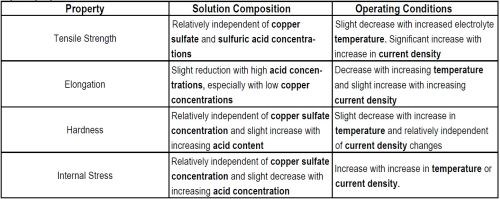
Table 7 - Trends relating solution composition and operating variables in copper sulfate electrolytes to deposit properties.
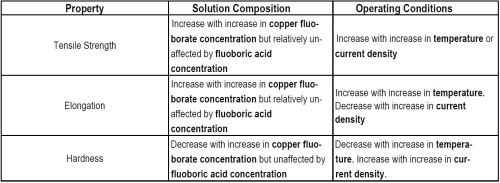
Table 8 - Trends relating solution composition and operating variables in copper fluoborate electrolytes to deposit properties.
Inorganic Impurities
The copper sulfate electrolyte has good tolerance towards many ionic impurities due its high acid concentrations. For instance, metals such as nickel, cobalt, zinc and iron will tend to build-up in solution rather than being plated-out with the copper. At concentrations greater than 1000 mg/L, iron and nickel are reported to reduce the electrolyte conductivity and throwing power but these are high levels and are unlikely to be reached in well operated electroforming plants.
In fluoborate electrolytes, lead is of most concern as it is very soluble and can cause severe embrittlement in the electroform.
Organic impurities
Organic impurities in copper sulfate electrolytes are often more of a problem than inorganic impurities. They can influence the appearance and mechanical properties of the electroforms and the sources of contamination are similar to those described for nickel electrolytes, e.g., tank linings, resists, plastic mandrels, additive breakdown products. Their removal is also similar. Treatment with activated carbon is normally sufficient, although in severe cases a hydrogen peroxide treatment may also be required.
Additives
All organics are not detrimental to the properties of copper electrodeposits. In fact, a wide range of organic additives have been evaluated and used in copper sulfate electrolytes. For electroforming, additives are usually used to refine the grain size, improve mechanical properties such as tensile strength and hardness and provide smooth deposits. A thorough evaluation of the overall effect of additives should be made before a commitment is made to their use and in this regard, it is advisable to consider the use of proprietary additives when specific properties are required in the electroform.
Mechanical properties of gold
In most gold electroforms, the mechanical properties are not of major importance but nevertheless, there are certain requirements for hardness and wear resistance in some applications. In jewelry applications, the brilliance and tarnish resistance of gold are of great significance, and in some functional applications, advantage is taken of the exceptional corrosion resistance of gold by electroforming a thin flash of gold and backing it up with nickel or copper.
When alloying with copper and cadmium in proprietary electrolytes to produce 10, 14 and 18 caret gold for jewelry, as deposited hardness values of 430 VHN are possible, which can be reduced to 220 VHN by annealing. Essentially 24 carat gold, containing no more than 1% copper and deposited from the sulfite-based electrolytes, can have a hardness value of 250 VHN. Such deposits which have application in producing dental crowns and bridges are also required to have adequate strength and stability. Typical values for both tensile strength and yield strength are 460 MN/m2.
RELATED CONTENT
-
Plastics and Plating on Plastics [1944]
This republished 1944 AES convention paper presents an historic perspective of the early days of plastics in surface finishing - using them and plating on them, in the waning years of World War II. The discussion reviews the uses of plastics in plating equipment and processing at that time, as well as the coating of the plastics themselves, with accompanying application photos. You will note that today’s conventional plating-on-plastics processes lay far in the future. Surprisingly, CVD processes are discussed.
-
Electroplated Tin-Nickel Coatings as a Replacement for Nickel to Eliminate Nickel Dermatitis
This paper is a peer-reviewed and edited version of a paper delivered at NASF SUR/FIN 2013 in Rosemont, Ill., on June 12, 2013.
-
PFAS and the Surface Finishing Industry
NASF and its member companies have a long history of environmental stewardship, especially when it comes to PFAS.


















