Method Validation for Selecting Baseline Parts Cleaning Solvents
Method validation to replace cleaning solvent AK-225G.
Precision cleaning is a critical step in production processes in the medical field, food industry, electronics manufacturing, and aerospace and defense. However, with ever-tightening environmental regulation, the availability of many production-proven solvents is becoming limited.
In fact, regulation is forcing AK-225G, the current baseline precision cleaning solvent, to be phased out by 2015.
Featured Content
Historically, chlorofluorocarbon 113 (CFC-113) was the baseline cleaning solvent NASA used agency-wide until 1989 with the acceptance of the Montreal Protocol, an international treaty designed to protect the ozone layer by phasing out the production of numerous substances believed to be responsible for ozone depletion.
NASA White Sands Test Facility (WSTF) was involved in finding a replacement for CFC-113 through collaboration with the Department of Defense, NASA contractors, and solvent manufacturers. Solvents under consideration had to be effective at cleaning and compatible with oxygen (O2), materials and aerospace fluids.
AK-225G, classified as an environmentally sensible hydrochlorofluorocarbon (HCFC-225), was recognized as the new baseline solvent; however, it was a transitional solvent so it, too, was slated to be phased out. Since WSTF is dependent on AK-225G for precision cleaning validation processes, a replacement needs to be found before the solvent is no longer available.
Method for Selecting Baseline Solvents
To seek a new solvent, WSTF developed a standardized method for evaluating suitable replacement baseline solvents by creating a test process to contaminate and then clean test coupons using solvents under consideration as replacement baseline solvents. The test process was based on work done in the 1990s using American Society for Testing and Materials (ASTM) standards on how to test and revalidate new solvents.
Since the applicable ASTM standards are practices or guides, not methods, there are a lot of options for every step in the testing process. With so many available options and no written record, it wasn’t possible to know the exact steps followed in the past; however, the results of the 1990s tests were documented, so the process that WSTF developed was ensured to be as close to past studies as possible by comparing its results.
According to ASTM, the most important attribute to consider for a baseline solvent is that it is effective for cleaning. The next considerations are carcinogenicity and toxicity. The solvent cannot be a class 1 or class 2 ozone-depleting substance, or it will have limited availability in the future, and environmental regulations are constantly changing as new discoveries are made. Baseline solvents cannot be HAPs or VOCs, and the solvent does not have to be O2 compatible, but it cannot be flammable or combustible. In addition, the evaporation rate must be known; if the solvent evaporates before it can be used, it isn’t effective.
ASTM also had a variety of test method options to choose from to contaminate and then clean the coupons. The coupons can be contaminated using one contaminant at a time, or a mixture, applied in a variety ways, including a pipette, brush, spray, or dip, applied to either one or both sides of the coupon. Drying the contaminant on the coupon can be accomplished either by hanging the coupons or by laying them flat, but either method still requires oven baking.
Cleaning choices include following the manufacturers’ recommended use of the solvent, sonication, or spraying the solvent on the coupons. The temperature of the solvent can also vary. And each manufacturer recommends something different, which again adds variables to the testing process.
During WSTF’s testing, the choices were narrowed down to ensure consistency in results. Six solvents were tested for cleaning efficiency on coupons exposed to known contaminants. WSTF used the following steps to test the solvents:
White Sands Testing Facility Solvent Testing Steps
1. Clean the coupons (standard cleaning process)
2. Weigh the coupons (tare weight)
3. Contaminate the coupons
a) Analyze slurry (filter/nonvolatile residue (NVR))
b) Oven dry
4. Weigh the coupons (determine contaminant)
5. Clean the coupons (test solvent)
a) Analyze rinse (filter/NVR)
b) Oven dry
6. Weigh the coupons (residual contaminant)
7. Verify cleanliness of coupons (AK-225-G, verification solvent)
a) Analyze rinse (filter/NVR)
b) Oven dry
WSTF Experimental Testing Method
The coupons were cleaned using the WSTF standard cleaning process, which begins with precleaning using ultrasonic baths and a visual inspection. Cleanliness verification is performed using deionized water, methyl nonafluoroisobutyl ether (HFE 7100TM), or isopropyl alcohol (IPA) as a cleaning agent. The process is validated using the baseline solvent AK-225G. The coupons were weighed for a baseline weight.
The contaminant slurry, aka “witches brew,” was a mixture of pump/hydrocarbon oil, hydraulic oil, O2 system lubricant (Krytox), gauge fluid, silicone grease, dye penetrant particles, and iron powder 60 mesh particles. Both liquids and particles were used. Five batches of slurry mixtures were made with 1 gram of each contaminant added to 100 mL of AK-225 at a concentration of 70 mg/mL. This concentration was selected because 70 mg/mL was used historically. The batches were mixed in the ultrasonic for 10 min.
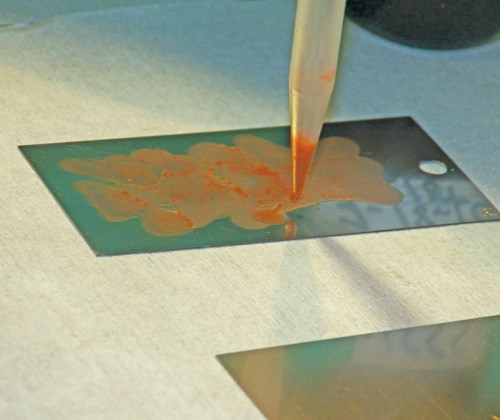
The coupons were contaminated with 600 μL of the contaminant slurry using a pipette to slowly drizzle the mixture onto one side of the coupon. After the coupons baked for one hour and cooled for one hour, they were weighed again, and then weighed again the next day to ensure the weight had stabilized.
A pressure vessel and a wand were used to spray the coupons with 100 mL of the test solvents at room temperature. After flushing with 100 mL of solvent, the solvent was captured to perform NVR analysis. The solvent captured from flushing the coupon was evaporated, and the residue left behind measured. The filters were sampled for a particle count to measure what was left on the coupon, and the filter papers were weighed to compare with the pretest weight.
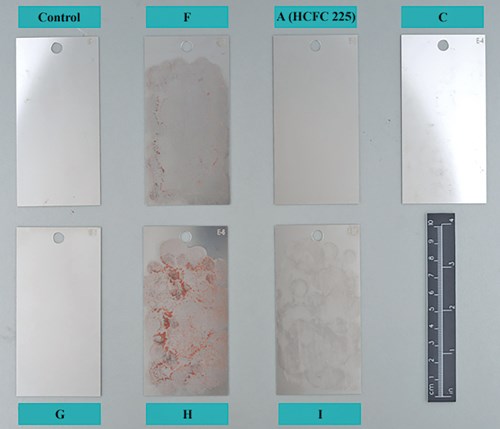
Coupon cleanliness was verified with AK-225G. Post cleaning showed that the coupons were not cleaned as well as initially indicated because when verified with AK-225G, residue was removed from the coupons.
Even though the additional validation step was not mentioned in the ASTM standards, WSTF added it to have a better understanding of what contaminants were left behind. The use of AK-225G in the validation step provided additional assurance that candidate solvents qualified as potential baseline solvents.
Test Method Results and Discussion
The current test method developed by WSTF can now be compared with the test method in the 1990s because results were consistent, which confirms that the approach was correct. However, data is limited since results were based on a small sample size, a problem that could be rectified with future testing. Getting a percent cleanliness efficiency number would allow us to rule out solvents that would never meet the criteria. The test data will support future precision cleaning and contamination control efforts.
Alongside the other baseline solvent candidates, AK-225 and CFC-113 were tested for comparison to past tests. Two of the solvents tested as potential baseline solvents were ruled out based on unsatisfactory test results, but three solvents showed possibility.
WSTF continues to seek a solution for the future baseline solvent.
Christina Y Piña Arpin and Rosella Sepulveda are with NASAs White Sands Test Facility. They can be reached at christina.y.pina.arpin@nasa.gov.
RELATED CONTENT
-
Cleaning Prior to PVD/CVD Coating
Determining the cleanliness and chemical de-coating of PVD/CVD layers.
-
The Hull Cell: Key to Better Electroplating - Part I
How to use it for planning, preventive maintenance and troubleshooting.
-
Top Reasons to Switch to a Better Cleaning Fluid
Venesia Hurtubise from MicroCare says switching to the new modern cleaning fluids will have a positive impact on your cleaning process.