Nanostructure of Porous Anodic Aluminum Oxide: Advances in Surface Engineering
Porous alumina membranes of anodic aluminum oxide (AAO) are widely used for the fabrication of various nanostructures and nanodevices. Over the last decade, nanowires, nanotubes and nanodot arrays, have been fabricated by the deposition of various metals, semiconductors, oxides and polymers inside the pores of AAO membranes. These structures are produced by changing the anodization conditions, during electrochemically self -ordering of AAO. One can obtain amorphous barrier-type oxides, crystalline barrier-type oxides or amorphous nanoporous oxides, but fabricating binary nanostructures is challenging. Here, we review methods to achieve diverse binary nanostructure arrays, including material, dimension and morphology.
#research #surfin
Editor’s Note: This paper is a peer-reviewed and edited version of a paper delivered at NASF SUR/FIN 2019, in Rosemont, Illinois on June 4, 2019 in Session 7, Advances II: Research. A pdf of this paper can be accessed and printed HERE. The original presentation delivered by Dr. Ventura is available HERE.
ABSTRACT: Porous alumina membranes of anodic aluminum oxide (AAO) are widely used for the fabrication of various nanostructures and nanodevices. Over the last decade, many materials, including nanowires, nanotubes and nanodot arrays, have been fabricated by the deposition of various metals, semiconductors, oxides and polymers inside the pores of AAO membranes. Access to these structures is achieved by changing the anodization conditions, such as current, voltage and type of electrolyte during electrochemically self -ordering of AAO. Numerous metals are subjected to anodic oxidation. Several physico-chemical effects and properties in the solid state involve nanoscale interactions between adjacent materials and morphologies. As a result, one can obtain amorphous barrier-type oxides, crystalline barrier-type oxides or amorphous nanoporous oxides. Currently highly-ordered nanoporous anodic aluminum oxide (AAO) arrays of binary nanostructures can generate intimate interactions between different sub-components, but fabricating binary nanostructures is challenging. Here, we propose a concept to achieve diverse binary nanostructure arrays with high degrees of controllability for each of the sub-components, including material, dimension and morphology.
Featured Content
Introduction: Anodization of aluminum has become one of the most popular processing methods to form porous structures with pore diameters ranging from about 10 to over 200 nm. The aluminum anodization process leading to a porous structure is usually performed in electrolytes containing sulfuric, oxalic or phosphoric acid. The structural features of the porous alumina film include pore diameters, interpore distance and porosity and strongly depend on the chosen electrolyte and anodizing potential.
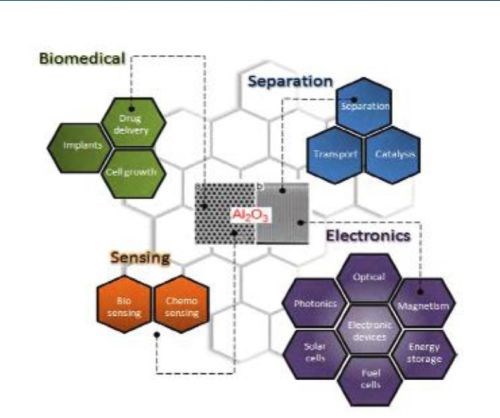
Figure 1 - Typical AAO structures and major applications for nanostructured materials.
Porous alumina membranes of anodic aluminum oxide (AAO) are widely used for the fabrication of various nanostructures and nanodevices. Over the last decade, many materials, including nanowires, nanotubes and nanodot arrays, have been fabricated by the deposition of various metals, semiconductors, oxides and polymers inside the pores of AAO membranes (Fig. 1).
Nanoporous substrates such as porous silicon, nanoporous anodic alumina (NAA), titania nanotube arrays and track-etched porous polymer membranes have been commonly employed as substrates for advanced sensing devices. NAA processes provide unique structural, chemical, optical, thermal and mechanical properties and biocompatibility besides controllable geometry and exploitable surface chemistry.
Ordered porous structures from the molecular to the macro level are widely used in nature. Indeed, these pore structures, with their elegant and intricate designs, have played pivotal roles in many biological processes. These processes include the transport of nutrients through the cell wall, selective transport of small or specific molecules or solutes, energy or charge transport, ion exchange, signaling and many other activities.
Ordered AAO stands out due to its properties, such as chemical, thermal stability, hardness and high surface area. Over the past decade, we have witnessed the emergence of various applications based on AAO membranes, such as molecular separation, chemical-biological sensing devices, cell adhesion, catalysis, energy storage and drug delivery vehicles. Recent advances in fabrication procedures toward structural modification and the generation of AAO with complex pore geometries, resulting in branched, multilayered, modulated and hierarchically complex pores architectures, are presented here.
Surface modification and nanoporous AAO
AAO nanostructuring is an active research area focused on the development of new fabrication concepts, to prepare more complex pore structures with properties suitable for diverse applications (Fig. 2). We can expect more exciting future developments in this field. Template synthesis of metals, semiconductor and polymer nanowires and nanotubes appear to be the primary motivation for these studies. However, these sophisticated AAO structures should find application in other areas, including molecular separation and in optics, for example as narrow-band filters.
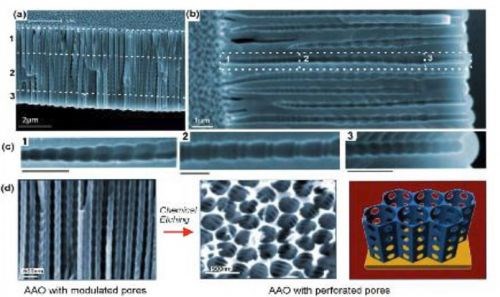
Figure 2 - (a-c) SEM images of AAO with multilayered pore architectures with different pore shapes and structural modulation fabricated by multiple cyclic anodization in 0.1M phosphoric acid with three successive galvanostatic anodization steps by three different cyclic signals; (d) AAO with periodically perforated pores (nanopores with nanoholes) by chemical etching.
Previously, we described recent advances in approaches for the structural engineering of AAO to generate new properties and applications. In this section, we will discuss current research on tuning surface properties of AAO using different surface modification strategies. The surface modifications and functionalization of AAO are expected to significantly expand the scope for applications of AAO-based materials. Furthermore, the effective combination of structural engineering and surface manipulation will underpin a number of emerging applications.
The AAO surface is insulating and suffers from chemical instability in acidic environments, which is a disadvantage for some applications. This limitation can be overcome by changing the surface properties and by adding new surface functionalities. The rich content of hydroxyl groups on the AAO surface allow them to be easily modified with organic molecules with the desired functionality.
The surface modification techniques that have been explored to improve surface properties and add new functionality to AAO can be divided into two groups: (a) wet chemical synthesis and (b) gas-phase techniques. Wet chemical approaches include self-assembly processes (silanes, organic acids and layer-by-layer deposition), polymer grafting, sol-gel processing as well as electrochemical and electroless deposition. Subsequent modifications of the thus introduced functionality with biomolecules of nanoparticles can be carried out.
Gas-phase surface modification techniques used for the surface modification of AAO include thermal vapor metal deposition, chemical vapor deposition (CVD), plasma processing, polymerization and atomic layer deposition (ALD). Combinations of these techniques on AAO have also been described, showing the ability to tune surface functionalities and properties of AAO for specific applications (Fig. 3).
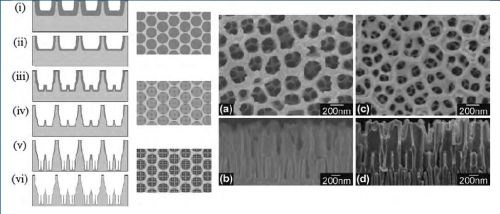
Figure 3 - (Left) Schematic diagram of the sequential fabrication steps of three-tiered branched AAO: (i and ii) first step, first-tier pore anodization and thinning of barrier layer; (iii and iv) second step, second tier formation at reduced potential followed by thinning of the barrier layer; (v and vi) third step, formation of third-tiered pores at further reduced anodization potential and final pore widening; (Middle) corresponding top views of all tiers. (Right) SEM microscopy of the resulting pore structures: (a and b) Top and cross-sectional views of two-tiered branched AAO prepared by combined anodization in 0.3M phosphoric acid (130V) and 0.15M oxalic acid (80V) followed by thinning of the barrier layer; (c and d) Top and cross-sectional views of the three-tiered branched AAO prepared by combined anodization in 0.3M phosphoric acid (130V), 0.15M oxalic acid (80V) and 0.15M oxalic acid followed by thinning of the barrier layer.
Complex internal pore architectures
AAO membranes with complex internal pore architectures hold considerable prospects for the development of advanced molecular separation and sensing platforms for use as templates for the fabrication of nanostructured nanowires and nanotubes with unique magnetic, electrical and optical properties. Several approaches have been explored to design modulated pore channels and architectures which include: multi-step anodization, where the type of electrolyte or electrolyte concentration is changed after each step; changing the electrolyte temperature during anodization; varying the applied voltage or current during anodization; or combining anodization and chemical etching.
It is well established that pore diameters in AAO are dependent on the nature of the electrolyte and the applied voltage used during anodization, so the simplest way to alter internal pore geometries would be to change these two parameters during etching.
This work sparked extensive interest in the fabrication of hierarchically Y-branched structures. The multiple branched pore structure was fabricated by a combination of reducing the applied voltage by a factor of 1/n, (n = the number of branches), changing the electrolyte after each anodization step and thinning the barrier layer after each anodization step at the pore bottom by chemical etching. The anodization potential in several steps and applied chemical etching after each step to fabricate AAO membranes with 3D multi-tiered branch nanostructures are shown schematically in Fig. 4. Three-tiered branched AAO having an average pore diameter of 275 nm, branching into four 125-nm sub-pores in the second tier and four 55-nm sub-pores in the third tier was demonstrated.
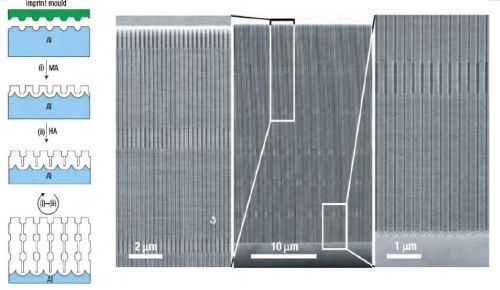
Figure 4 - Long-range ordered AAO membranes with modulated pore diameters: (Left) Scheme for the fabrication of porous alumina with modulated pore diameters by a combination of mild and hard anodization on a pre-patterned aluminum substrate; (Right) SEM micrographs showing the cross-section of the prepared AAO with modulated pore diameters. Magnified cross-section images of the top and bottom parts of the membrane are shown on both sides of the central image.
The variation of anodization conditions by changing electrolytes allows the fabrication of AAO membranes with periodic diameter-modulation in the pore structures combined with the conventional mild anodization (MA) and hard anodization (HA) processes to achieve periodic modulation of the pore diameter. The length of each segment was controlled by varying the time of the anodization process. This approach was extended to form shaped pores with nano-funnel geometry. Stepwise anodization processes with two different electrolytes were used, first with phosphoric acid and followed by oxalic acid. Subsequently, an inverted nano-funnel was obtained by switching the electrolyte sequence.
More recently a method called “pulse anodization” where both MA and HA processes were applied in the same electrolyte has been developed. The method is based on applying long low current pulses (MA regime) followed by short high current pulses (within the HA regime).
Although these methods demonstrate the ability to create different pore diameters across the AAO structure, it does not allow control over the shape of the pore. A new anodization process has been reported to achieve improved control over internal pore shape. Called “cyclic anodization,” the concept is based on slow and oscillatory changes of anodization conditions ranging from the MA to the HA regimes. By changing the period of the current, control over the geometry of the pore structure was achieved and AAO with asymmetrical (ratchet-type) and symmetrical (circular) periodic pore geometries, with different lengths, periodicity and gradient were fabricated. Periodic variations in the pore diameter led to photonic crystal behavior in AAO as observed in optical photographs and transmission spectra. The color of the sample and the diffraction peak position in the transmission spectra could be easily controlled by using a chemical post-etching step. Similarly, AAO membranes with conical holes were fabricated. The conical holes were produced by repeating and alternating anodization at 40V and chemical etching with 5% phosphoric acid solution at 20-30°C. Nano-sculpting of AAO membranes with hierarchical pore structures was also accomplished. Three different successive cyclic anodization steps were applied, first a cycle with a double-profiled cycle and lastly, a series of triangular cycles. SEM images of the resulting AAO membrane showed hierchical and multi-modulated pore structures consisting of three stacked porous layers. Chemical etching led to periodically perforated pores (nanopores with nanoholes).
Organosilane modification of AAO
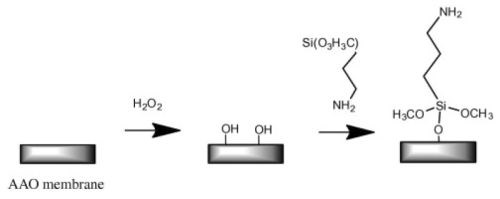
Figure 5 - Common schematic route of silanization used for surface modification of AAO membranes.
Organosilane modification of AAO, i.e., chemical modification using silanization, is a simple and effective method of controlling the wettability and adsorption properties of AAO (Fig. 5). Wide varieties of substituted silanes are commercially available, and their attachment has a profound effect on the properties of AAO. Some of these silane modifiers are widely used as coupling agents or linkers to immobilize other biomolecules, polymers, nanoparticles, DNA cell and suspended lipid bilayers on AAO surfaces.
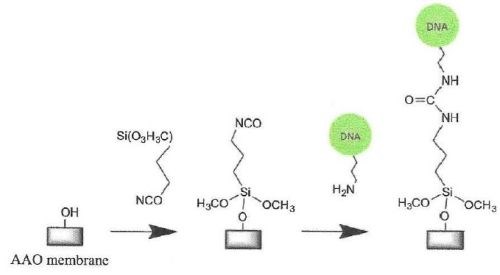
Figure 6 - Silanization of a hydrolylated AAO surface with isocyanato-propyl triethoxysilane and subsequent immobilization of amino-terminated DNA.
Silane-coated surfaces have also been used to suspend lipid bilayers over the pores of AAO membranes functionalized with a grafted polymer layer (Fig. 6).
Preparation of biometallic Au-Pd nanotubes has also been demonstrated using this approach. Accumulation in the wet stage and solidification of nanoparticles upon drying promoted the formation of multiwall metallic nanotubes inside the AAO. In a different approach, AAO functionalized perfluorotriclosilane was reported to help minimize the adhesion between the pore wall and polyacrylate nanofibers formed using pressure impregnation and photo crosslinking inside nanopores
Accumulation in the wet stage and solidification of nanoparticles upon drying promoted the formation of multiwall metallic nanotubes inside AAO. In a different approach, AAO functionalized with 1H, 2H perfluordecyltrichlorosilane was reported to help minimize the adhesion between the pore wall and polyacrylate nanofibers formed using pressure impregnation and photo-crosslinking inside nanopores.
Our group has recently demonstrated multifunctional and multilayered surface modification of AAO nano-pores with distinctly different internal and external surface properties. The multilayered surface functionalities were successfully fabricated by combining a series of anodization and silanization cycles with different silanes, for instance, pentafluorophenyldimethylchloro-silane (APTES), (3-aminopropyl) triethoxysilane (APTES) and N-triethoxy-silylpropyl-(0-polyethyleneoxide) urethane (PEG-silane), achieving a range of functionalities and wettabilities (Fig. 7).
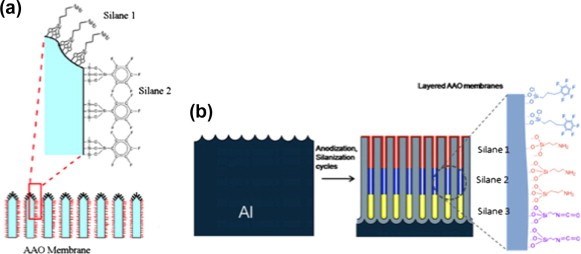
Figure 7 - (a) Schematic of the opening of an AAO membrane wth different silane functionality on the very top of the membrane and the inside of the pores. (b) Schematic of anodization and silanization cycles to produce an AAO membrane with multiple silane layers.
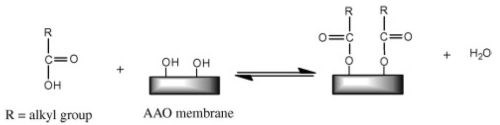
Figure 8 - Reaction scheme of AAO membrane with n-alkanoic acid.
This approach also enabled control over the thickness of individual layers inside the pore matrix, and to selectively bind gold nanoparticles on amino functionalized layers. Selective transport experiments with hydrophobic and hydrophilic molecules on pores with hydrophobic and hydrophilic layers subsequently verified the effectiveness of our modification approach and showed an elegant approach to tune chemical separation properties of AAO membranes.
In contrast, fusion of lipid vesicles inside AAO nanopores has been achieved using silane-coated membranes, as illustrated in Fig. 9(a). Pioneering work led to multi-lamellar lipid nanotubes by exposure of the AAO to multi-lamellar vesicles or extruder deposition (Fig. 9(b)). By the latter technique, the wall thickness of the result, can be controlled. The formation of these architectures can be confirmed by means of spin-labeling electron paramagnetic resonance(EPR), solid state NMR spectroscopy and fluorescence microscopy using fluorescent lipids.
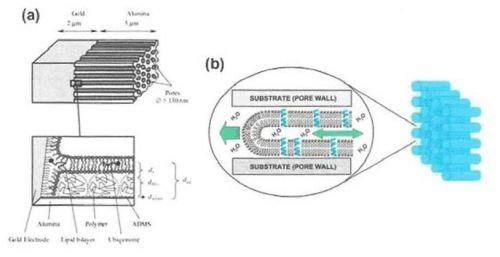
Figure 9 - (a) Top: Structure of the AAO membrane with an underlying Au layer as a host for a self-assembled lipid bilayer; Bottom: Enlarged view of the pore bottom showing the self-assembled lipid bilayer along the pore wall on polymer-functionalized surfaces; (b) Schematic of single lipid nanotubes (LNT’s) propagating inside an AAO pore by capillary action.
A different approach involved a deposited tethered lipid bilayer membrane inside AAO channels. The lipid bilayer membrane was prepared following step-by-step techniques inside the pores with the last step being polyethylene glycol (PEG)-triggered fusion on the surface-attached liposomes. The pore bottom of AAO was first gold-coated and functionalized with an undecanethiol, whilst the pore walls were modified by (3-aminopropyl) triethoxysilane (APTES). The system was incubated with liposomes which were then triggered to fuse using PEG. The authors demonstrated the importance of incorporating the lipid membrane inside the pores to prevent membrane dewetting. These studies showed that, by integration of lipid bilayers and nano-pore structures, it is possible to design sensitive biomimetic nano-channel-based sensing devices and use them to study protein membrane interactions.
Layer-by-layer deposition
Layer-by-layer deposition is a simple, versatile and inexpensive technique, which involves construction of polyelectrolyte multilayer films by alternate dipping of a substrate into polyelectrolyte solutions of alternating charge (Fig. 10(a)). The film thickness can be easily controlled within a nanometer scale. The resulting layers can be further functionalized with biomolecules or nanoparticles. The most popular polyelectrolytes used for deposition are polyacrylic acid (PAA), polyallylamine hydrochloride (PAH), polystyrene sulfonate (PSS) and polydiallydimethiylammonium chloride (PDADMAC).
The selectivity of Mg+2 rejection can be enhanced by increasing the charge of the terminated PAH layer by means of increasing ionic strength in the PAH deposition solution.
This process is followed by immobilization of antibodies on the carboxylic groups of the PAA layers using carbodiimide coupling (Fig. 10(b)). The resulting coating resisted nonspecific protein adsorption. Charged polyelectrolytes on AAO membranes also allowed the immobilization of citrate-stabilized gold nanoparticles on PEMs under retention of the nanoparticle catalytic activity.
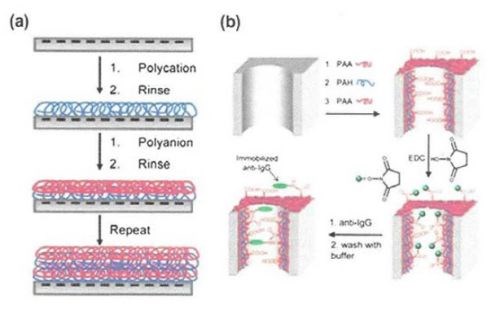
Figure 10 - (a) Schematic of of an AAO membrane pore modified by adsorption of two polyelectrolytes of opposing charge; (b) schematic of an AAO membrane pore modified by adsorption of polyelectrolytes followed by carbodiimide coupling of an antibody.
Comparative study with application of click chemistry in nanoparticles
Here, we compare click chemistry with more traditional chemistries in the pharmaceutical, industrial and medical areas. Click chemistry is a class of biocompatible small molecular reactions commonly used in bioconjugation, allowing the joining of substrates of choice with specific biomolecules and a reporter molecule. Not limited to biological conditions, the concept of a click reactions has been used pharmacological and various biomimetic applications. However, they have been notably useful in the detection, localization and qualification of biomolecules.
The term “click chemistry” was coined K.B. Sharpless in 1998, and was first fully described by Sharpless, H. Kolb and M.G. Finis of the Scripps Research Institute in 2001.
In order for this technique to be useful in biological systems however, click chemistry must run at or near biological conditions which produce little and ideally no toxic byproducts, have preferably single and stable products under the same conditions, and proceed quickly to high yield one-pot synthesis. The objective of this strategy is to improve the efficiency of a chemical reaction, such as the Standinger reaction, a chemical reaction of an azide with phosphine or phosphite:
R3P + RN3 à 3P + NR + N2.
The Huispen 1,3 dipolar cycloaddition is a chemical reaction between a 1,3 dipolar and a dipolarophite to form a five-membered ring. The earliest 1,3 dipolar-cycloadditions have been modified and optimized for such reaction conditions.
Research in the field concerns not only understanding and developing new reactions and repurposing and reunderstanding known reactions. The research also seeks to expand the methods used to incorporate reaction partners into living systems, engineering novel reaction partners, and developing applications for bio-conjugation.
During development of the nanoparticles, click chemistry is generally used to attach biological ligands to the surface of nanoparticles (Fig. 11). An antibody is one of the more favored ligands to increase bonding to a specific cell line.
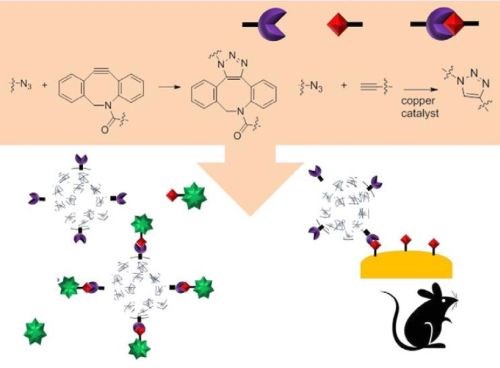
Figure 11 - Schematic of the usage of click chemistry during nanoparticle synthesis and its targeting in vivo.
In 2017, Liu, et al. developed a folate-receptor targeted surface, for enhanced Raman scattering (SERS) nanoprobes based on click chemistry. They synthesized hollow gold nanoparticles to enhance the Raman signals, and modified them with azide-labeled 5,5-dithiobis 2-nitrobenzoic acid (DTNB), a Raman active molecule, by an interaction between thiol and the gold surface. The azide groups on the surface of nanoparticles were then conjugated to folate bicyclo derivatives, a kind of strain-promoted alkyne by click chemistry. The signals were not observed in the case of using azide-modified nanoparticles without folate or folate-saturated KB cells, showing that click chemistry enabled easy conjugation of folate to the nanoprobe without perturbation of its Raman imaging property.
In the same year, Chen applied click chemistry to enhance the targeting efficiency of nanogels to overcome multi-drug resistance (MDR). The synthesized biodegradable nanogel was composed of hydroxyethyl-methacrylamide-oligoglycolates-derivatized poly(hydroxyethyl methacrylamide-co-AzEMAm)-Gly-HEMAm), and doxorubicin (DOX) was loaded in it. After gelation, the surface of the nanogel-containing azide groups was decorated with folic acid (FA)-polyethylene glycol (PEG)-BCN via copper-free click chemistry (Fig. 12). The nanogel backbone has pH-sensitive hydrazine linkages between the methacrylamide polymer and DOX, which can lead to the fast release of DOX in cancer cells.
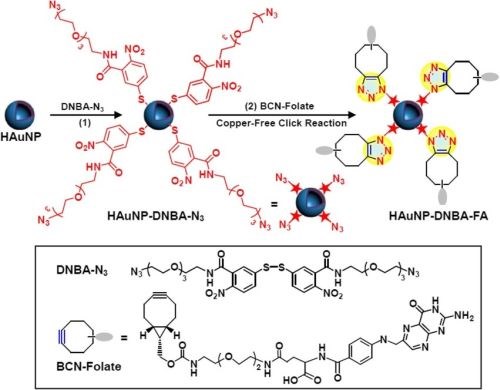
Figure 12 - Modification of SERS nanoprobes with folate by copper-free click chemistry between azide and BCN for cancer cell imaging.
The nanogel showed a faster cellular uptake in the FA receptor-positive cell line than in the FA receptor-negative cell line due to receptor-mediated endocytosis. Demonstrating the successful conjugation of FA-PEG to nanogels by click chemistry, DOX was rapidly released in cytosol with the low pH environment and accumulated in the nuclei. Furthermore, the FA-modified nanogel showed a lower resistance index compared to other non-targeted formulations, and efficient killing of DOX-resistant cells was observed, bypassing the drug efflux pumps of MDR.
Drug delivery
A range of nanoscale materials has been explored in recent years for drug delivery applications to address the problems associated with conventional drug therapies, such as limited drug solubility, poor biodistribution, lack of selectivity and unfavorable pharmacokinetics. Among them are nanoporous and nanotube carriers, due their unique features, such as low fabrication costs, controllable pore /nanotube structure, tailored surface chemistry and high surface area.
Implantable systems for local delivery of therapeutics, based on porous platforms fabricated by electrochemical processes including pSi, AAO and TNT have been widely explored. Good mechanical stability, chemical inertness, biocompatibility, controllable pore size and pore volumes, along with tunable surface chemistry, have made AAO to be an excellent platform for loading large amounts of drugs and facilitating their controlled release.
To address the problem of sustained release of poorly soluble drugs from implants, Simovic demonstrated extended drug release based on applying a thin plasma polymer film on the top of AAO membrane after drug loading (Fig. 13(a)). A plasma polymer layer with different thicknesses deposited on AAO allowed control over the pore diameter and hence the rate of drug release (Fig. 13(b)). It was therefore possible to achieve favorable zero order release kinetics from AAO implants by controlling the deposition of a plasma polymer layer. More recently, this group explored using AAO as therapeutic implants for the elution of drug nanocarriers using several polymer micelles as model nanocarriers.
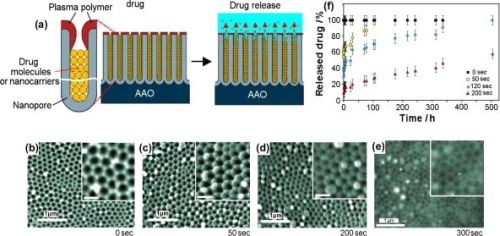
Figure 13 - (a) Schematic of plasma polymer modification of an AAO platform loaded with drug or drug nanocarriers (polymer micelles) to achieve controlled and extended drug release; (b-e) SEM images at the top surface of the AAO porous layer modifier with allylamine plasma polymer using deposition times of (b) 0 sec; (c) 50 sec; (d) 200 sec and (e) 300 sec. The scale bars in the inserts are 200 nm; (f) controlled release of model drug vacomycin from plasma-modified AAO during 500 hr of drug release.
The concept of switchable drug release from AAO has been recently demonstrated by Jeon, designing an electrically responsive AAO membrane based on an electropolymerized dodecylbenzenesulfonate-doped polypyrrole (PPy/DBS) coating on the outer part of the AAO membrane. The pore size of the PPy/DBS AAO membrane could be reversibly actuated by the electrochemical state and pulsatile release of the model protein drug (FITC-BSA) (Fig. 14). Considering these results, accurate drug dosage control, tailored to a specific patient state would be possible, which is relevant to emergency therapy of acute angina pectoris and migraine, hormone-related diseases and metabolic syndromes.
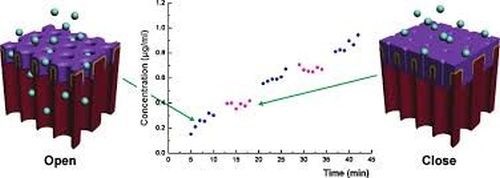
Figure 14 - Schematic of an electrically-responsive electropolymer-coated AAO membrane showing the reversible change of pore size (and the drug release rate) between oxidation and reduction states.
Summary and conclusions
Over the past several years, significant progress has been made regarding structural engineering and surface modification of nanoporous AAO materials. Much of this progress has been application-driven. In this review, we have drawn together innovative approaches on controlling and designing the structural growth of AAO with different sizes, arrangements, structures, geometries and pore architectures. Access to these structures is achieved by changing anodization conditions such as current, voltage and the type of electrolyte during the electrochemically self-ordering of AAO.
An excellent consistency between experimental results and theoretical predictions of the interpore distance was observed for the highest studied anodizing time and the highest ethanol content in the anodizing electrolyte.
We introduced the recent application of click chemistry in nanoparticle research. During modification of biological ligands on the surface of nanoparticles, the intrinsic properties and functions of both ligands and nanoparticles need to be preserved. Click chemistry is suitable for these purposes and is used to attach ligands to nanoparticles easily.
In this review paper, we have shown:
- Applications for nanostructured materials for biomedical-separation-sending and electronics,
- Pores of AAO membranes modified by adsorption on two polyelectrolytes of opposing charge,
- Electrically-responsive electro-polymer-coated AAO membranes showing reversible change of pore size between oxidation and reduction states,
- Silanization with AAO silane multilayers and
- Polyelectrolytes of pores in AAO membranes modified by adsorption of two polyelectrolytes coupling of an antibody.
Researchers are also pushing the boundaries of molecular separations using AAO with pores of controlled shape and size, internal surface modification, and are exploring the effect of external parameters, such as pH, flux, concentration gradient and ionic strength.
Fabrication of complex AAO nanostructures combined with even greater control over surface functionality is expected to lead to unique nanostructures and nano-devices with unprecedented functional properties for the next generation devices, including the exploration of their application with a focus on different research areas ranging from medicine to material science and electronics.
Bibliography
1. T. Kondo, H. Masuda & K. Nishio, “SERS in Ordered Array of Geometrically Controlled Nanodots Obtained Using Anodic Porous Alumina,” J. Phys. Chem. C, 117 (6), 2531-2534 (2012).
2. G. Gauglitz & J. Ingenhoff, “Design of New Integrated Optical Substrates for Immuno-Analytical Applications,” Fresenius .J. Anal. Chem., 349 (5), 355-359 (1994).
3. T. Kumeria, L. Parkinson & D. Losic, “Bioinspired Microchip Nanoporous Interferometric Sensor for Sensing and Biosensing Applications,” Micro Nanosyst., 3 (4), 290-295 (2011).
4. G. Gauglitz, “Direct Optical Detection in Bioanalysis: An Update,” Anal. Bioanal. Chem., 398 (6) 2363-2372 (2010).
5. K.S. Mun, et al., “A Stable, Label-Free Optical Interferometric Biosensor Based on TiO2 Nanotube Arrays,” ACS Nano., 4 (4), 2070-2076 (2010).
6. H.C. An, J.Y. An & B-W. Kim, “Improvement of Sensitivity in an Interferometry by Controlling Pore Size on the Anodic Aluminum Oxide Chip Pore-Widening Technique,” Korean J. Chem. Eng., 26 (1) ,160-164 (2009).
7. R. Dronov, et al., “Nanoporous Alumina-Based Interferometric Transducers Ennobled,” Nanoscale, 3 (8), 3109-3114 (2011).
8. G. Macias, et al., “Gold-Coated Ordered Nanoporous Anodic Alumina Bilayers for Future Label-Free Interferometric Biosensors,” ACS Appl. Mater. Interfaces, 5 (16), 8093-8097 (2013).
9. M. Pumera, Nanomaterials for Electrochemical Sensing and Biosensing, CRC Press, Boca Raton, FL, 2014.
10. E. Ekanayake, D.M. Prethichandra & K. Kaneto, “Polypyrrole Nanotube Array Sensor for Enhanced Adsorption of Glucose Oxidase in Glucose Biosensors,” Biosens. Bioelectron, 23 (1), 107-113 (2007).
11. G. Jeon, et al., “Electrically Actuatable Smart Nanoporous Membrane for Pulsatile Drug Release,” Nano Lett., 11 (3),1284-1288 (2011).
12. R.E. Sabzi, K. Kant & D. Losic, “Electrochemical Synthesis of Nickel Hexacyanoferrate Nanoarrays with Dots, Rods and Nanotubes Morphology Using a Porous Alumina Template,” Electrochim. Acta, 55 (5), 1829-1835 (2010).
13. V. Rai, et al., “Ultrasensitive, cDNA Detection of Dengue Virus RNA Using Electrochemical Nanoporous Membrane-Mased Biosensor,” PLoS ONE, 7 (8): e 42346 (2012).
14. J.Q. Ang, B.T.T. Nguyen, C-S. Toh, “A Dual K+/Na+ Selective Prussian Blue Nanotubes Sensor,” Sensors and Actuators B: Chemical, 157 (2), 417-423 (2011).
15. K. Kant, et al., “Characterization of Impedance Biosensing Performance of Single and Nanopore Arrays of Anodic Porous Alumina Fabricated by Focused Ion Beam (FIB) Milling,” Electrochim. Acta, 139, 225-231 (2014).
16. L. Juhasz & J. Mizsei, “Humidity Sensor Structures with Thin Film Porous Alumina for On-Chip Integration,” Thin Solid Films, 517 (22), 6198-6201 (2009).
17. Z. Jin, et al., “A Novel Porous Anodic Alumina Based Capacitive Sensor towards Trace Detection of PCBs,” Sensors and Actuators B: Chemical, 157 (2), 641-646 (2011).
18. L.H. Cohen & A.I. Gusev, “Small Molecule Analysis by MALDI Mass Spectrometry,” Anal. Bioanal. Chem., 373 (7), 571-586 (2002).
19. R. Nayak, et al., “Dual Desorption Electrospray Ionization-Laser Desorption Ionization Mass Spectrometry on a Common Nanoporous Alumina Platform for Enhanced Shotgun Proteomic Analysis,” Anal. Chem., 80 (22), 8840-8844 (2008).
20. A. Sen, et al., “Use of Nanoporous Alumina Surface for Desorption Electrospray Ionization Mass Spectrometry in Proteomic Analysis,” Biomed. Microdevices, 10 (4), 531-538 (2008).
21. Y. Wada, T. Yanagishita & H. Masuda, “Ordered Porous Alumina Geometries and Surface Metals for Surface-Assisted Laser Desorption/Ionization of Biomolecules: Possible Mechanistic Implications of Metal Surface Melting,” Anal. Chem., 79 (23), 9122-9127 (2007).
22. I. Goubaidoulline, G. Vidrich & D. Johannsmann, “Organic Vapor Sensing with Ionic Liquids Entrapped in Alumina Nanopores on Quartz Crystal Resonators,” Anal. Chem., 77 (2), 615-619 (2005).
23. Y.L. Rao & G. Zhang, “Enhancing the Sensitivity of SAW Sensors with Nanostructures,” Current Nanoscience, 2 (4), 311-318 (2006).
24. Z. Yang, et al., “Piezoelectric Urea Biosensor Based on Immobilization of Urease onto Nanoporous Alumina Membranes,” Biosens. Bioeelectron., 22 (12), 3283-3287 (2007).
25. J. Runge & A. Pomis, ”Continued Development of Anodic Oxide Finishes for Aluminium: Evaluation of Selected Mechanical Properties,” Proc. AESF Aerospace Aircraft Forum 2002.
26. M.E. Davis, “Ordered Porous Materials for Emerging Applications,” Nature, 417, 813-821 (2002).
27. K. Liu, X. Yao & L. Jiang, “Recent Developments in Bio-Inspired Special Wettability,” Chem. Soc. Rev., 39 (8), 3240-3255 (2010).
28. S. Majd, et al., “Applications of Biological Pores in Nanomedicine, Sensing and Nanoelectronics,” Opin. Biotechnol., 21 (4), 439-476 (2010).
29. P.L. Stroeve & N. Ileri, “Biotechnical and Other Applications of Nanoporous Membranes,” Trends Biotechnol., 29 (6), 259-266 (2011).
30. K. Nielsch, et al., “Self-Ordering Regimes of Porous Alumina: The 10 Porosity Rule,” Nano Letters, 2 (7), 677-680 (2002).
31. R.C. Furneaux, W.R. Rigby & A.P. Davidson, “The Formation of Controlled-Porosity Membranes from Anodically Oxidized Aluminium,” Nature, 337, 147-149 (1989).
32. I. Woo & K. Jae-Cheon, “Highly Ordered Porous Alumina with Tailor-Made Pore Structures Fabricated by Pulse Anodization,” Nanotechnology, 21 (48) 485304 (2010).
33. L. Yi, et al., “Novel AAO Films and Hollow Nanostructures Fabricated by Ultra-High Voltage Hard Anodization,” Chem. Commun., 46 (2), 309-311 (2009).
34. Z. Fan, et al., “Nanoporous Anodic Aluminium Oxide Membranes with 6-19 nm Diameters Formed by a Low Potential Anodizing Process,” Nanotechnology, 18 (34), 345302 (2007).
35. K. Lee, Y. Tang & M. Ouyang, “Self-Ordered Controlled Structure Nanoporous Membranes using Constant Current Anodization,” Nano Letters, 8 (12), 4624-4629 (2008).
36. G.E. Thompson & G.C. Wood, “Porous Anodic Film Formation on Aluminium,” Nature, 290, 230-232 (1981).
37. M.J. Barela, et al., “Fabrication of Patterned Arrays with Alternating Regions of Aluminium and Porous Aluminium Oxide,” Electrochem. Solid-State Letters, 8 (1) C4-C5 (2005).
38. M. Gowtham, et al., “Controlled Fabrication of Patterned Lateral Porous Alumina Membranes,” Nanotechnology, 19 (3), 035303 (2008).
39. V. Grasso, et al., “Nanostructuring of a Porous Alumina Matrix for a Biomolecular Microarray,” Nanotechnology, 17 (3), 795 (2006).
40. M. Harada, et al., “Anodic Porous Alumina Masks with Checkerboard Pattern,” Appl. Phys. Express, 3 (1) 015001 (2010).
41. L. Vellerman, et al., “Structural and Chemical Modification of Porous Alumina Membranes,” Microporous and Mesoporous Materials, 126 (1-2), 87-94 (2009).
42. Z.D. Hendren, J. Brant & M.R. Wiesner, “Surface Modification of Nanostructured Ceramic Membranes for Direct Contact Membrane Distillation,” J. Membrane Sci., 331 (1-2), 1-10 (2009).
43. D.J. Odom, L.A. Baker & C.R. Martin, “Solvent-Extraction and Langmuir-Adsorption-Based Transport in Chemically Functionalized Nanopore Membranes,” J. Phys. Chem. B, 109 (44), 20887-20894 (2005).
44. S.W. Lee, et al., “Transport and Functional Behavior of Poly(ethylene glycol)-Modified Nanoporous Alumina Membranes,” Nanotechnology, 16 (8), 1335-1340 (2005).
45. C. Hobler, I.J. Bakowsky & M. Keusgen, “A Functional Immobilization of Semiconductor Nanoparticles (Quantum Dots) on Nanoporous Aluminium Oxide,” Phys. Status Solidi A, 207 (4), 872-877 (2010).
46. G. Demirel & F. Buyukserin, “Surface-Induced Self-Assembly of Dipeptides onto Nanotextured Surfaces,” Langmuir, 27 (20), 12533-12538 (2011).
47. J.B. Largueze, K. EI Kirat & S. Morandat, “Preparation of an Electrochemical Biosensor Based on Lipid Membranes in Nanoporous Alumina, Colloids and Surfaces B - Biointerfaces, 79 (1), 33-40 (2010).
48. T.D. Lazzara, et al., “Phospholipids as an Alternative to Direct Covalent Coupling: Surface Functionalization of Nanoporous Alumina for Protein Recognition and Purification,” J. Colloid Interface Sci., 366 (1), 57-63 (2012).
49. A.M.M. Jani, et al., “Dressing in Layers: Layering Surface Functionalities in Nanoporous Aluminum Oxide Membranes,” Angew Chem. Int. Ed., 49 (43), 7933-7937 (2010).
50. E.K. Schmitt, et al., “Electrically Insulating Pore Suspending Membranes on Highly Ordered Porous Alumina Obtained from Vesicle Spreading,” Soft Matter, 4 (2), 250-253 (2008).
51. E.K. Schmitt, C. Weichbrodt & C. Steinem, “Impedance Analysis of Gramicidin D in Pore-Suspending Membranes,” Soft Matter, 5 (17), 3347-3353 (2009).
52. Q. He, et al., “Self-Assembly of Composite Nanotubes and their Applications,” Current Opinion in Colloid Interface Sci., 14 (2), 115-125 (2009).
53. R. Barbery, et al., “Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties and Applications,” Chem. Rev., 109 (11), 5437-5527 (2009).
54. S.T. Grajales, et al., “Effects of Monomer Composition on CO2-Selective Polymer Brush Membranes,” Chem. Mater., 22 (13), 4026-4033 (2010).
55. M.A.C. Stuart, et al., “Emerging Applications of Stimuli-Responsive Polymer Materials,” Nat. Mater., 9 (2), 101-113 (2010).
56. C. Song, et al., “pH-Sensitive Characteristics of Poly(Acrylic Acid)-Functionalized Anodic Aluminium Oxide (AAO) Membranes,” J. Membrane Sci., 372 (1-2), 340-345 (2011).
57. B. Ma, et al., “Hierarchically Structured Anatase Nanotubes and Membranes,” Microporous and Mesoporous Materials, 124 (1-3), 163-168 (2009).
58. M-H. Park, et al., “Silicon Nanotube Battery Anodes,” Nano Lett., 9 (11), 3844-3847 (2009).
59. A. Zhang, et al., “Synthesis of Silica Nanotubes with Orientation Controlled Mesopores in Porous Membranes via Interfacial Growth,” Chem. Mater., 24 (6), 1005-1010 (2012).
60. W. Shi, et al., “Lysine-Attached Anodic Aluminum Oxide (AAO)-Silica Affinity Membrane for Bilirubin Removal,” J. Membrane Sci., 349 (1-2), 333-340 (2010).
61. J.D. Berrigan, et al., “Protein-Enabled Layer-by-Layer Syntheses of Aligned, Porous-Wall, High-Aspect-Ratio TiO2 Nanotube Arrays,” Adv. Funct. Mater., 21 (9), 1693-1700 (2011).
62. M. Cheng, et al., “Au Nanoparticle Arrays with Tunable Particle Gaps by Template-Assisted Electroless Deposition for High Performance Surface-Enhanced Raman Scattering,” Nanotechnology, 21 (1), 015604 (2010).
63. Y. Yu, et al., “Gold Nanotube Membranes have Catalytic Properties,” Microporous and Mesoporous Materials, 153, 131-136 (2012).
64. L. Velleman, D. Losic and J.G. Shapter, “The Effects of Surface Functionality Positioning on the Transport Properties of Membranes,” J. Membrane Sci., 411-412, 211-218 (2012).
65. K. Pitzchel, et al., “Controlled Introduction of Diameter Modulations in Arrayed Magnetic Iron Oxide Nanotubes,” ACS Nano, 3 (11), 3463-3468 (2009).
66. K. Marianna, et al., “Ta2O5 and TiO2-Based Nanostructures Made by Atomic Layer Deposition,” Nanotechnology, 212 (3), 035301 (2010).
67. C. Marichy, M. Bechelany & N. Pinna, “Atomic Layer Deposition of Nanostructured Materials for Energy and Environmental Applications,” Adv. Mater., 24 (8), 1017-1032 (2012).
About the author

Dr. Xavier Albort Ventura earned his Ph.D. in Chemical Engineering Industrial from the University of Barcelona Spain. He is a member of the Barcelona International Trade Fair and has organizing responsibility for the Environment Technical Meetings and at the Eurosurfas show. He is an electroplating consultant for more of 50 European companies from various countries, including Portugal, France, Spain, Italy and Germany. Dr. Ventura has been an active presenter at SUR/FIN, having given papers since 1987. He has also been an active participant at Interfinish, presenting papers and conducting workshops since 1984. He has published more than 200 papers in Spain, the United Kingdom, France, the United States and Brazil, and has won a number of national and international awards in the United States. He receivedd the Sam Wyman Memorial Award at SUR/FIN 2004 in Chicago for the Best Light Metals paper, “Sealing Method for Anodizing Aluminum and Hard Anodizing and a Test to Determine the Seal Quality of Aluminum.”
*Corresponding author
Xavier Albort Ventura, Professor and Consultant
University Politecnic of Barcelona Spain Research and Development
C. Carabela.la La Niña, 22-24 bis
Barcelona, Barcelona Spain 08017
Phone: 34-93-2054936
Mobile: 34-63-9721065; 34-60-1370304
E-mail: xavieralbort@ing-mediamb.com
RELATED CONTENT
-
How to Apply the 720 Rule to Current Density Anodizing
What can you tell me about the 720 Rule as it applies to current density anodizing? Plating expert Sjon Westre, Ph.D., from Chemeon, answers this question.
-
Zinc Electroplating
Choosing the best process for your operation.
-
Preventing Anodizing Cathodes from Turning Red
While the red color may not be desirable, anodizing expert Drew Nosti says it poses no particular problem to a successful anodizing process.


















