NASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters: Full Progress Report
This NASF-AESF Foundation research project has been ongoing since April 2019 but was unavoidably interrupted by the SARS CoV-2 pandemic of 2020-21. This report (1) re-introduces the project and (2) covers all of the work thru June 2021. The project focuses on an electrochemical, destructive treatment strategy for the remediation of relevant PFASs in electroplating wastewater. The overall objective is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater.
#pollutioncontrol #nasf
by
Brian Chaplin*
Department of Chemical Engineering
University of Illinois at Chicago
Chicago, Illinois, USA
Featured Content
Editor’s Note: NASF-AESF Foundation research project report R-120 has been ongoing since April 2019 but was unavoidably interrupted by the SARS CoV-2 pandemic of 2020-21. This report (1) re-introduces the project and (2) covers all of the work thru June 2021 on this project at the University of Illinois at Chicago. A printable PDF version of this report is available by clicking HERE.
Part 1 - Introduction to the Project Work
1. Introduction
Per- and polyfluoroalkyl substances (PFASs) are a class of chemicals that have unique properties that impart oil and water repellency. As a result, PFASs have been used as coatings for textiles, paper products and consumer packaging, as well as for metal plating1 and in various other industries (e.g., semiconductors, automotive, construction).2 As a result of the widespread use of PFASs and their resistance to destructive treatment and biodegradation, these compounds are ubiquitous in the environment.3 There is growing evidence of their toxicity to humans.4 In response, the Environmental Protection Agency (EPA) has issued a combined Health Advisory Level for perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS) of 70 ng/L, and several states have endorsed standards for these and other PFASs.4 However, due to its ability to act as mist suppressant, PFOS has been used for metal finishing and electroplating applications in the form of tetraethylammonium PFOS salt (CAS #56773-42-3).5 New formulations have been introduced that utilize other polyfluoralkyl substances (e.g., 6:2 fluorotelomer sulfonate (6:2 FTS)) in place of PFOS. Given the health concerns and emerging regulatory standards of PFASs, reliable low-cost methods for destructive removal of PFOS and other PFASs are greatly needed.6
The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. The REM is a patent-pending technology that utilizes a conductive ceramic electrode material with micron-sized pores to electrochemically oxidize or reduce contaminants in a flow-through operation. Specific technical objectives associated with the proposed work include:
- Development of REMs for destructive PFAS removal in synthetic electroplating wastewater.
- Determination of the optimal operational mode.
- Calculation of energy requirements for the REM-based system and compare to those determined for GAC adsorption and other technologies.
Achieving these objectives will provide the necessary data to determine if the REM system is competitive with other treatment options and thus will allow for the pursuit of further funding from industry and other funding agencies. Specific technical questions are stated below.
Question 1: Can adsorbent materials be added to REMs to produce next generation REMs with enhanced sorption capacities for PFAS?
Question 2: What is the best mode of operation for optimal REM performance for PFAS removal?
Question 3: Will the REMs be a technically effective and cost-efficient remediation strategy for PFAS-containing electroplating wastewater?
2. Related work
In this work, we are investigating the technical and economic feasibility of using REMs for treatment of synthetic electroplating wastewater containing PFAS. A schematic of one possible configuration of a REM unit is shown in Fig. 1(a). The concentric porous cathode/anode tubes shown in the figure are made of solid Ti4O7 (25 mm cathode OD; 200 mm long; pore size ~1.0 μm; porosity ~30%). Depending on the treatment objective or the need to remove carbonate scale from the cathode, the anode and cathode can be switched by changing the polarity of the direct current (DC) voltage, without any physical manipulation of the device.
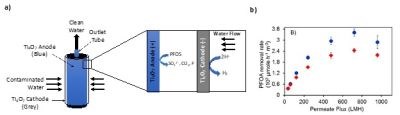
Figure 1 - (a) Schematic diagram of a concentric cylindrical reactive electrochemical membrane (REM) used for the treatment of PFAS in liquid streams. The flow configuration is outside-in, dead end, filtration mode; (b) results for removal rates at 2.9 VSHE for PFOS (red diamonds) and PFOA (blue circles) oxidation in the REM as a function of permeate flux (electrolyte 100 mM K2HPO4). Starting concentrations for PFOA and PFOS were both 10 μM.8
The REM was used to degrade PFOA and PFOS in single-pass mode as a function of permeate flux (L/m2/hr (LMH)). The removal rates were calculated and are shown versus the permeate flux in Fig. 1(b). These results are very promising compared to other treatment methods. For example, at a permeate flux of 720 LMH the removal rates were 2436 and 3415 μmol/m2/hr for PFOA and PFOS, respectively.8 This permeate flux corresponded to a 3.8 sec residence time in the reactor and achieved 50 and 40% removal of PFOA and PFOS, respectively.8 Optimal energy consumption per log removal of 5.1 kWh/m3 for PFOA and 6.7 kWh/m3 for PFOS was achieved.8 The rate constants for PFOA and PFOS removal (607 and 210 per hr, respectively) in our work were over 100 times higher than previously reported for other treatment technologies, with energy consumptions that were a factor of ten lower.8
3. Technical approach
The work plan consists of REM synthesis, a series of bench-scale experimental studies that will determine optimal operating conditions for PFAS oxidation in synthetic electroplating wastewater samples, and a preliminary energy cost assessment. Experimental parameters that will be explored include: (1) adsorption capacity, (2) necessary residence time in the reactor, (3) needed membrane surface area per water volume treated and (4) energy usage (kWh/m3 water treated). The results will provide proof-of-concept that the REM technology is suitable for the treatment of PFOS in electroplating wastewater.
4. Relationship to NASF goals
This project is focused on the removal of PFAS from electroplating wastewater. There is little treatment of these wastewater streams at electroplating facilities, and typically these wastewaters are directly discharged to the municipal wastewater treatment plant. However, due to the growing concern over PFOS and other PFASs in the environment, there has been much further scrutiny of this practice by industries. A key example is in the state of Michigan, where regulators are considering imposing standards on industrial wastewaters containing PFASs. The proposed work will focus on an economical and technically viable treatment solution, which can enable PFAS treatment. Therefore, it is expected that there would be considerable interest across fields in the electroplating community and beyond.
Part 2 - 1st and 2nd Quarter Report (April-September 2019)
Summary
Work was conducted on the synthesis of reactive electrochemical membranes (REMs), both with and without sorbent materials (Task 1), and preliminary PFOS oxidation tests in synthetic electroplating wastewater (Task 2). Experiments that were conducted in a complimentary SERDP-funded project (SERDP project # ER18-1491) indicated that the addition of carbonaceous materials (i.e., multiwalled carbon nanotubes (MWCNTs)) and powder activated carbon (PAC) significantly increased the sorption capacity of PFOA.9 However, the carbon content within the REMs was not electrochemically stable. Therefore, the use of carbon-Ti4O7 composite REMs for the simultaneous adsorption and oxidation of PFOS was determined to not be a feasible strategy for PFOS remediation. The use of carbon-free Ti4O7 REMs for PFOS oxidation was tested in a single-pass flow-through reactor with a liquid residence time of ~11 sec. Results indicated that PFOS (feed concentration of 40 μM) was degraded by 65% at 2.9 VSHE and by 81% at 3.6 VSHE. These results have established proof of concept that the REM is a viable technology for PFOS removal from electroplating wastewater. Future work will focus on optimizing the operating conditions, investigating the removal of PFOS from real electroplating wastewater, and testing the performance of the REM for the oxidation of PFOS replacements (e.g., 6:2 FTS, GenX).
Carbon-REM electrochemical stability
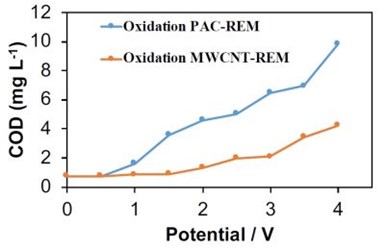
Figure 2 - COD permeate concentrations for PAC-REM and MWCNT-REM as a function of the anodic potential.
In a complimentary SERDP-funded project the electrochemical stability of the PAC-REM and MWCNT-REM materials were tested as a function of the applied anodic potential (SERDP project # ER18-1491).9 These experiments were conducted in a flow-through reactor and effluent solution from the reactor was collected as a function of potential. Results are summarized in Fig. 2. Results for the PAC-REM indicated that it began to oxidize at applied potential values higher than 0.5 VSHE.
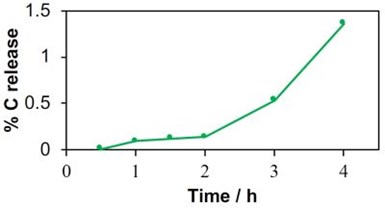
Figure 3 - Percent carbon leaching for the MWCNT-REM at a potential of 3.0 VSHE as a function of time.
The chemical oxygen demand (COD) value increased continuously from 0.76 mg/L at 0.5 VSHE to 9.81 mg/L at 4.0 VSHE. Results for the MWCNT-REM were more promising, but carbon leaching was still observed. For example, COD values in the effluent reached as high as 4.2 mg/L at an applied voltage of 4.0 VSHE. These results indicated that the composite REMs were not stable under anodic conditions. To determine if the MWCNT-REM stability would improve with longer operation times, an additional experiment was conducted at 3.0 VSHE over a four-hour period. Results are shown in Fig. 3 and indicate that stability of the MWCNT-REM deteriorated over time. Effluent COD levels were as high as 455 mg/L after 4 hours, which amounted to approximately 1.5% of the total carbon content. Thus, it is apparent that these composite REMs are not appropriate for PFAS oxidation. Therefore, other strategies are needed to remove and treat PFAS. Upstream adsorption followed by downstream oxidation is being explored.
Electrostatic PFAS adsorption
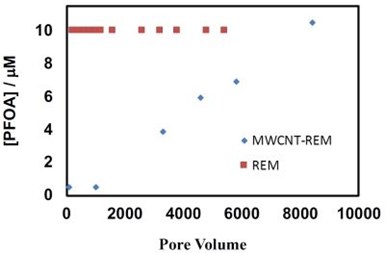
Figure 4 - PFOA effluent concentrations as a function of pore volumes processed in the REMs with an applied potential of 0.5 VSHE. Influent PFOA concentration of 10 μM.
Electrostatic adsorption of PFOA was investigated to determine if an adsorption step prior to electrochemical oxidation could be used to concentrate PFASs prior to electrochemical oxidation. Two flow-through electrode materials were investigated, including a Ti4O7 REM and a MWCNT-Ti4O7 REM. These REMs were polarized at an anodic potential of 0.5 VSHE, which was a potential where the MWCNT-REM was stable. Results are shown in Fig. 4 and indicate that the Ti4O7 REM did not provide any adsorption capacity. However, significant adsorption was observed for the MWCNT-REM. PFOA breakthrough occurred at over 2000 pore volumes and effluent concentrations did not reach influent concentration until approximately 10000 pore volumes. These results indicate that an electrosorption step prior to electrochemical oxidation could prove to be an effective treatment strategy for PFAS remediation. Ongoing work is focused on integrating the adsorption and oxidation steps.
Electrochemical PFOS oxidation
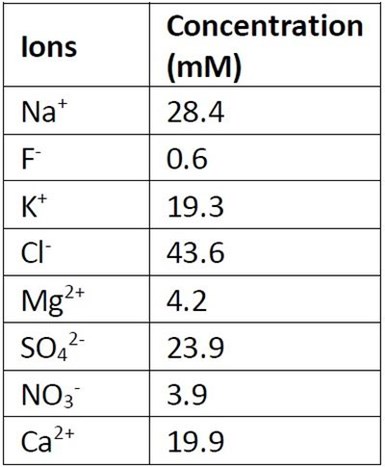
Table 1 - Composition of the synthetic electroplating wastewater. PFOS concentration was 40 μM.
The electrochemical oxidation of 40 μM PFOS in a synthetic electroplating wastewater was tested using a Ti4O7 REM in a single-pass flow-through mode. The liquid residence time in the reactor was ~11 sec and oxidation was tested at anodic potentials of 2.9 and 3.5 VSHE. The composition of the synthetic wastewater is listed in Table 1. Results indicated that PFOS was degraded by 65% at 2.9 VSHE and 81% at 3.6 VSHE. These results provide proof of concept that the REM is a viable technology for the elimination of PFOS from electroplating wastewater. Future work will focus on optimizing operating conditions, oxidation of real electroplating wastewaters, and investigation of the oxidation of PFOS replacements (e.g., 6:2 FTS, F-53B).
Theoretical reactivity of PFOS replacements
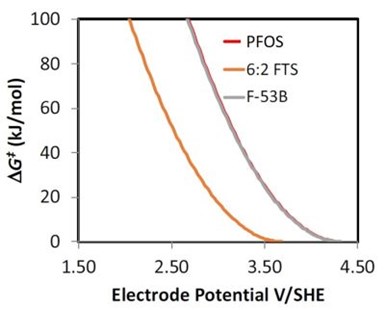
Figure 5 - DFT results showing the free energy of activation (∆G‡ ) versus the electrode potential for the direct oxidation reaction of 6:2 FTS, F-53B and PFOS.
Work has begun on determining the reactivity of PFOS replacements (e.g., 6:2 fluorotelomer sulfonate (6:2 FTS) and F-53B). Initial work focused on investigating the potential dependent reactivity of these compounds using density functional theory (DFT) modeling. A summary of these results is shown in Fig. 5, where the free energy of activation (∆G‡) versus the electrode potential is plotted for the direct oxidation reaction of 6:2 FTS, F-53B and PFOS. The direct oxidation reaction was modeled, as it has been shown to be the rate limiting step for PFOS oxidation. According to the results shown in Fig. 5, the reactivity of F-53B to the direct oxidation reaction should be similar to PFOS. However, results for 6:2 FTS show a much lower ∆G‡ value than both F-53B and PFOS at a given electrode potential. These results suggest that 6:2 FTS should react readily via electrochemical oxidation. Future work is planned to investigate the reactivity of both F-53B and 6:2 FTS.
Part 3 - Pandemic Interim (October 2019-September 2020)
Work was significantly slowed by the COVID-19 pandemic during this period. The laboratories were shut down on March 15, 2020, and reopened on June 15, 2020 at limited capacity. Due to the limited lab capacity (~50%), laboratory work was suspended. Work was conducted by PI Chaplin to review the literature related to electrochemical PFAS oxidation, and a review manuscript was published in Environmental Science and Technology.10 In addition, a new Ph.D. student was assigned to the project and experimental work resumed on October 28, 2020. For obvious reasons, the project period was extended by one year.
Part 4 - 3rd Quarter Report (October-December 2020)
Summary
With the labs at 100% capacity, laboratory work this quarter was focused on the development of a tubular reactor to test PFAS oxidation. Work was also conducted by PI Chaplin to review the literature related to electrochemical PFAS oxidation, and a review manuscript was published in Environmental Science and Technology.10
Tubular Reactor Setup
A schematic of the tubular reactor is shown in Fig. 6. It is comprised of a tubular Ti4O7 reactive electrochemical membrane (REM) and a centrally located stainless steel rod. The reactor was initially tested for PFOA oxidation in a 100% recycle mode (recycle of both feed and permeate) in a 240 mM NaClO4 background electrolyte. The NaClO4 background electrolyte was chosen as a non-electroactive electrolyte that could mimic the solution conductivity of electroplating wastewater. The results are shown in Fig. 7 and indicate >95% removal was achieved over the 9-hour experiment. The data fit a second-order model, with a R2 value of 0.98. Further work is underway to investigate PFAS oxidation in synthetic wastewater samples.

Figure 6 - Photo of tubular REM reactor module
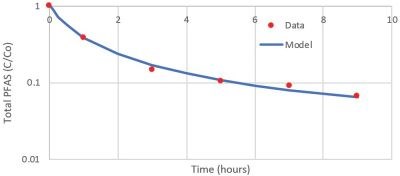
Figure 7- Total PFAS oxidation in 100% recycle experiment.
Part 5 - 4th Quarter Report (January-March 2021)
Summary
Laboratory work this quarter continued to focus on the development of a tubular reactor to test PFAS oxidation. Work was specifically focused on overcoming challenges related to current collector corrosion. It was found that plugging the pores on the ends of the membrane using a sol-gel method and utilizing niobium as a current collector overcame problems with corrosion.
Current Collector
During the course of long-term PFAS oxidation experiments (> 8 hours), it was found that the stainless-steel current collector that was used to make electrical contact with the porous reactive electrochemical membrane (REM) began to release iron into solution. Since the electrode is porous, the current collector was in direct contact with the electrolyte as it permeated through the pores. To overcome the corrosion of the current collector, two strategies were utilized. First, different metals were investigated as suitable current collectors. Second, the pores on the edge of the REM were sealed to provide an area for connection of the current collector where the electrolyte was not present. For the first task, we tested several metals (e.g., Au-plated stainless steel, Ti, Nb and Ta). It was found that both Nb and Ta were the most resistant to corrosion, and ultimately Nb was chosen because it is cheaper than Ta.
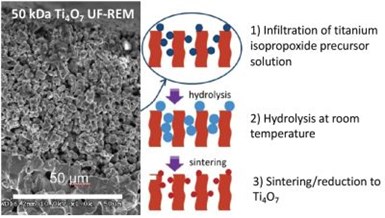
Figure 8 - Procedure for dealing REM pores.
The second task (i.e., sealing the pores) was accomplished by using a sol-gel method. Sealing of the pores was accomplished using a dip coating procedure, that is shown schematically in Fig. 8. The Ti4O7 REM was dip coated in a titanium isopropoxide sol gel solution. The sol was allowed to hydrolyze at room temperature and then sintered / reduced at 900°C in flowing H2. The hydrolysis step allowed for the growth of the TiO2 coating, and the heat treatment provided adhesion and reduction of the TiO2 coating to Ti4O7. The procedure shown in Fig. 8 was repeated for three cycles in order to completely seal the pore.
Part 6 - 5th Quarter Report (April-June 2021)
Summary
Work this past quarter focused on testing of the reactor that was developed in past quarters for electrochemical oxidation of poly- and per- fluoroalkyl substances (PFAS) using a tubular Magnéli phase titanium sub-oxide (Ti4O7) reactive electrochemical membrane (REM). The oxidation experiments were performed in synthetic solutions, using a crossflow setup in full recycle mode. The synthetic solutions were prepared by spiking perfluorooctanoic acid (PFOA) into a background electrolyte that has an ionic strength that is representative of industrial electroplating wastewaters (i.e., 240 mM NaClO4). The solution was subjected to electrochemical oxidation at a constant current density (30 mA/cm2) for between 5 to 9 hours, achieving > 90% PFAS removal.
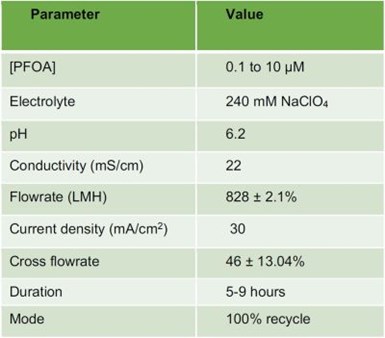
Table 2 - Operating parameters of PFAS oxidation experiments.
The reaction rate constants were found to be approximately second-order with respect to total PFAS concentration, indicating that interactions between adsorbed intermediates is likely involved in the reaction mechanism. Additionally, shorter-chain PFASs were observed to form and degrade throughout the experiment. Therefore, future work will focus on improving electrocatalytic activity to minimize the production of these compounds.
Electrochemical oxidation experiments
The operating conditions for the electrochemical oxidation experiments are shown in Table 2 (above) and results are shown in Fig. 9.
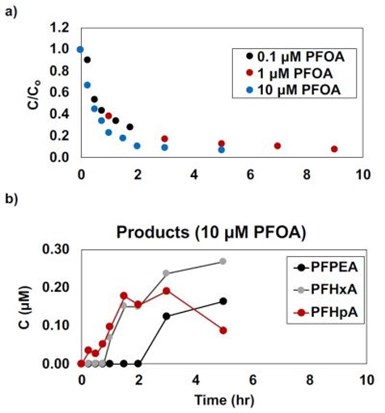
Figure 9 - (a) PFOA oxidation results, (b) product formation from 10 μM PFOA oxidation experiment.
Three initial PFAS concentrations were investigated (i.e., 0.1, 1.0, and 10 μM) to determine the effect of initial concentration on removal. It was found that rapid removal was observed for the initial two hours of the experiment (i.e., > 80% removal) followed by a slow removal to > 90% over the duration of the experiment (Fig. 9a). All three concentrations showed the production of short chain compounds, which is in line with the literature. The concentrations of PFHpA, PFHxA, and PFPeA are shown in Fig. 9b for the 10 μM initial PFOA concentration and are produced at < 2.7% of initial PFOA. The trends for these short chain compounds indicate that they are both being produced and degraded during the experiment.
Kinetic modeling of the results is shown in Fig. 10 and indicates that the reactions are second-order with respect to total PFAS concentration.
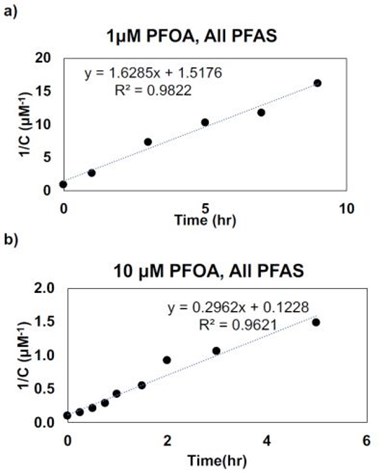
Figure 10 - Second-order kinetic modeling for total PFAS for initial PFOA concentration of (a) 1.0 μM and (b) 10 μM.
Only the 1.0 and 10 μM PFAS concentrations are shown because the 0.1 μM concentration had considerable noise in the data due to the very low product concentrations. The consequences of the second-order kinetics are that while high PFAS concentrations can be degraded very rapidly, low PFAS concentrations will require extended electrolysis times. These results suggest that PFAS oxidation should be coupled to an up-concentration step, which may be accomplished using sorbents or membranes. Future work will thus focus on evaluating this strategy and investigating the effect of REM properties to enhance PFAS reaction rates.
References
1. Z.W. Du, et al., “Efficient Adsorption of PFOS and F53B from Chrome Plating Wastewater and their Subsequent Degradation in the Regeneration Process,” Chem. Eng. J., 290, 405-413 (2016).
2. Interstate Technology and Regulatory Council, “History and Use of Per- and Polyfluoroalkyl Substances (PFAS),” (2017).
3. I. Ross, et al., “A Review of Emerging Technologies for Remediation of PFASs,” Remediation Journal, 28 (2), 101-126 (2018); https://cswab.org/wp-content/uploads/2018/05/Emerging-Technologies-for-Remediation-of-PFAS-Ross-2018.pdf (last accessed 07/30/2021).
4. Interstate Technology and Regulatory Council, “Regulations, Guidance and Advisories for Per- and Polyfluoroalkyl Substances (PFAS),” (2018).
5. Environmental Protection Agency, “PFOS Chromium Electroplater Study, U.S. Environmental Protection Agency, Region 5,” (2009): https://www.in.gov/idem/ctap/files/plating_chromium_pfos_study.pdf (last accessed 07/30/2021).
6. J.B. Weiss, et al., “PFAS Analysis in Water for the Global Monitoring Plan of the Stockholm Convention: Set-up and Guidelines for Monitoring,” UNEP Chem. Branch, (2016); https://wedocs.unep.org/bitstream/handle/20.500.11822/29682/Analisis_PFAS_En.pdf?sequence=1&isAllowed=y; pp. 1-26 (last accessed 07/30/2021).
7. Y. Jing, L. Guo and B.P. Chaplin, “Electrochemical Impedance Spectroscopy Study of Membrane Fouling and Electrochemical Regeneration at a Sub-Stoichiometric TiO2 reactive Electrochemical Membrane,” J. Membrane Sci., 510, 510-523 (2016).
8. T.X.H. Le, H. Haflich, A.D. Shah and B.P. Chaplin, “Energy-Efficient Electrochemical Oxidation of Perfluoroalkyl Substances Using a Ti4O7 Reactive Electrochemical Membrane Anode, Environmental Science & Technology Letters, 6, (8), 504-510 (2019).
9. B.P. Chaplin, and A. Shah, Reactive Electrochemical Membrane (REM) Reactors for the Oxidation of PFAS-Impacted Water, Final Report, SERDP Project ER18-1491; 2020.
10. J. Radjenovic, N. Duinslaeger, S.S. Avval and B.P. Chaplin, “Facing the Challenge of Poly- and Perfluoroalkyl Substances in Water: Is Electrochemical Oxidation the Answer?,” Env. Sci. & Technol., 54 (23), 14815-14829 (2020). Abstract, access and supporting information: https://pubs.acs.org/doi/pdf/10.1021/acs.est.0c06212.
About the author

Dr. Brian P. Chaplin is Associate Professor in the Department of Chemical Engineering, at the University of Illinois at Chicago. He holds a B. Civil Engineering (1999) and an M.S. (2003) in Civil Engineering from the University of Minnesota and a Ph.D. in Environmental Engineering (2007) from the University of Illinois at Urbana-Champaign.
*Dr. Brian Chaplin
Associate Professor
Dept. of Chemical Engineering
Dept. of Civil, Materials, and Environ. Eng.
Institute for Environmental Science and Policy
University of Illinois at Chicago
154 EIB
929 W. Taylor St., Chicago, IL 60607
Office: (312) 996-0288
Mobile: (217) 369-5529
E-mail: chaplin@uic.edu
RELATED CONTENT
-
Smut and Desmutting
Question: I am new to this industry and have heard about smut and desmutting operations.
-
Nitric Acid Passivation and EH&S Impact
I am looking for safety/environmental requirements to set up a nitric acid cleaning and passivation system.
-
NOx Scrubbing Technology Breakthrough
This paper presents research findings and practical results that address the treatment of the problematic greenhouse gases nitrogen oxides (NOx) and sulfur dioxide (SO2).


















