Opportunism! The 21st William Blum Lecture
Opportunism, a fading principle of today's society? Not according to this AES Scientific Achievement Award winner. Fast-rate plating, new processes for alloy deposition, electroforming and several other areas offer potential for opportunists in plating and surface finishing. Mr. Safranek presented this text, the 21st William Blum Lecture, at AES Annual Conference in Milwaukee.
by William H. Safranek
Recipient of the 1979 William Blum AES Scientific Achievement Award
Crime-story buffs will recognize that the pursuit of opportunity in electroplating is like the conduct of fictional investigators who apply their skills to solving crimes. The leading characters in each category relentlessly pursue their objective - the elucidation of a mystery. In a broad, nonderogatory sense, they can be called opportunists because they successfully use their ability in a timely manner in response to the pressing needs of the time.
Featured Content
In this sense, each of my predecessors, starting with Dr. William Blum, was an opportunist (Fig. 1). Dr. Blum, in whose name this dissertation for the AES Scientific Achievement Award is presented, began his distinguished career at the National Bureau of Standards (NBS) by investigating a problem of electroforming copper printing plates at the government printing office. He quickly recognized that electroplating offered boundless opportunities for scientific study and began to devote all of his energy and considerable talent to research on electrodeposition.
In just a few years, Dr. Blum molded a strong group of scientists at the NBS, each member devoting his faculties to solving problems in electroplating. These actions demonstrate that Dr. Blum recognized a vital need and dedicated his ability to fulfill that need. He also was adept at inspiring others to seek an understanding of electrodeposition principles. The book on that subject which he coauthored with George Hogaboom was the first of its kind and assumed an important role in teaching electroplating principles to both practitioners and researchers.
Dr. A.K. Graham, the second recipient of the Scientific Achievement Award, also was an opportunist who recognized the need to apply engineering principles to electroplating. With the help of a prestigious group of highly qualified engineers, he compiled the Electroplating Engineering Handbook, first published [56] years ago. This handbook provided designers, purchasers and operators of electroplating equipment with a wealth of basic data on equipment aspects, which were sorely needed to promote the advancement of the electroplating industry.
Dr. Charles Faust, third winner of the achievement award, has been called an opportunist who exploited an error to create a new technology. Concluding a plating experiment and recognizing that he had attached the wrong lead to the anode, Dr. Faust astutely noted that the anode surface had become a little smoother and brighter. This observation prompted an investigation that culminated in the development of electropolishing.
A few years later, Dr. Faust developed the principles of electrochemical machining, which employs very high anode current densities and rapid solution flow to achieve a fast rate of metal removal from the workpiece. Shortly thereafter, while directing a team dedicated to finding ways of accelerating plating rates, he encouraged the group to switch the anode and cathode connections in a cell similar to that used for electrochemical machining. The results confirmed and extended the pioneering research of Dr. Andrew Wesley, fifth recipient of the award, who had previously combined rapid solution flow with high cathode current densities to achieve useful deposits at very fast rates. Several other opportunists now are taking a good look at prospective applications for this technology.
Other award-winners also were opportunists in at least one respect. For example, Dr. Abner Brenner recognized the need for a reliable nondestructive method of measuring thickness of electrodeposits and so applied magnetic principles to design and develop a portable, compact device that was widely adopted soon thereafter. Some observers have marked the development of the MagneGage* as the true beginning of specification plating.
Because thickness testers based on magnetic principles are incapable of distinguishing nonmagnetic coatings from nonmagnetic substrates, Boris Joffe recognized the need for another instrument that would satisfy the demands of what was then an emerging electronics industry. Pursuing a new principle, beta-ray backscatter, Mr. Joffe developed new, high-precision instruments of the type used today for a variety of purposes. As a result, specification plating with good thickness control advanced rapidly.
Each of these distinguished scientists cultivated opportunities to further our knowledge of electrodeposition. Like Sherlock Holmes, these sleuths collected the evidence, traced the elements, and cracked the case. And, while it is entirely appropriate to recognize past achievements, it perhaps is even more important to recognize that opportunities still abound.
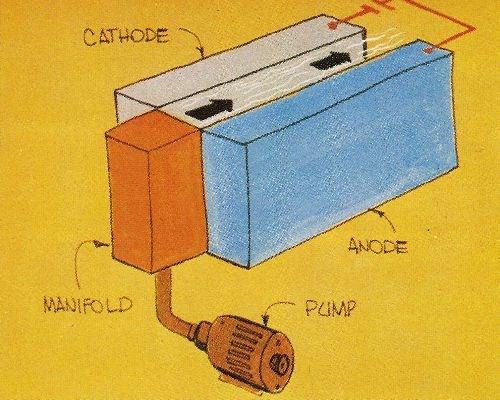
Several recent advances in technology pose opportunities that can be exploited. For example, further use can be made of fast plating techniques similar to that already developed for plating nickel and gold on continuous strip, subsequently used to fabricate electrical connectors. This development is an outstanding example of opportunism pursued by several teams of scientists and engineers. The reel-to-reel, selected-area plating processes used in the electronics industry have replaced slower, less reliable and more costly barrel plating operations. With these new processes, product performance is enhanced and gold usage is reduced simultaneously. Fast plating processes rely on rapid solution flow to achieve quality deposits at high current densities (Fig. 2).
Some new applications of fast plating techniques that could be adopted to reduce costs and/or improve product performance include the following:
• Sequentially depositing inexpensive, corrosion-resistant, solderable coatings such as tin-lead alloy on selected areas, and high-conductivity coatings such as gold on the contact areas of printed circuits, using gasketed manifolds to restrict solution flow to the selected areas.
• Fabricating circuitry by a process that transfers electroformed copper patterns to either rigid or flexible substrates and that uses proven adhesives (or good bonds in order to eliminate the waste associated with etching the copper.
• Cladding aluminum with copper to produce wire with high conductivity and surfaces with low contact resistance.
• Galvanizing one or both sides of continuous steel strip.
• Fabricating useful forms of metals such as continuous strip, tubing or wire by electrorefming (copper) or electrowinning (zinc) and eliminating energy-intensive melting processes.
• Depositing wear-resistant coatings such as chromium on cylindrical stock useful for piston rods and hydraulic rams.
• Producing a low-cost stainless steel by depositing chromium on one or both sides of a continuous strip (or wire) of low-carbon steel and subsequently diffusing the chromium by a rapid, controlled heating process.
• Depositing nickel and black chromium on a continuous strip of copper or aluminum to produce material easily fabricated into solar-collector panels.
• Plating liners with chromium to improve the wear life of piston engines and compressors.
• Fabricating ductile, electroformed iron foil for packaging purposes.
• Electropurifying plating baths such as nickel solutions to effectively and efficiently remove undesirable impurities like copper, iron and zinc.
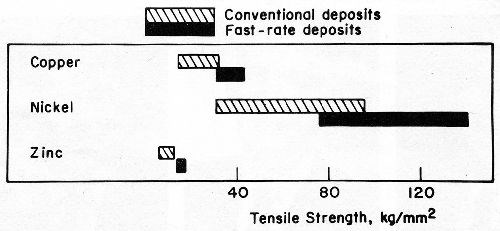
For each of the above applications, the use of methods established for electrodepositing metals at rates as high as 0.1 to 0.15 mm/min (Table 1) will conserve materials or energy and reduce space requirements and costs. Fast plating techniques with this capability offer electrodepositors and fabricators new tools for producing a myriad of products that could not be fashioned economically with conventional plating processes. Moreover, the properties of fast-rate deposits frequently are better than the properties of the corresponding metals deposited at conventional rates (Fig. 3).
Successful results depend on two key elements conferred by advanced engineering technology:
1. The rate of solution flow or cathode motion should range from 1.5 to 3 m/sec to promote good cathode efficiency and prevent undesirable nonmetallic inclusions.
2. The gap between cathodes and anodes should be in the range of about 2 to 5 mm in order to minimize energy use.
Metal | Solutiona | Practical current density, A/cm2 | Deposition rate, μm/min |
Chromium | 3.0M CrO3 | 6.2 | 20 |
Cobalt | 2.5M Co(BF4)2 | 6.2 | 125 |
Copper | 2.0M CuSO4 or Cu(BF4)2 | 3.1 | 75 |
Gold | 0.5M AuCN(citrate) | 0.3 | 18 |
Iron | 2.3M Fe(NH2SO3)2 | 6.9 | 150 |
Lead | 2.5M Pb(BF4)2 | 1.6 | 100 |
Nickel | 2.0M NiSO4 or NiCl2 | 3.0 | 60 |
Tinb | 1.0M Sn(BF4)2 | 3.0 | 140 |
Cadmiumc | 1.0m Cd(BF4)2 | 3.0 | 120 |
Zinc | 2.0M ZnSO4 | 3.0 | 80 |
aMetal salt solutions containing no additive agent. bData for 1.0- to 1.5-μm thick deposits on steel. cData for 13-μm thick coating. 1W.H. Safranek & C.H. Layer, Trans. Inst. Metal Finishing, 53, 121 (1975). | |||
The codeposition of a small amount of manganese with nickel improves its ductility at high temperatures, according to recent reports. Exploitation of this discovery is expected to yield improved electroformed and nickel-clad products intended for use at high temperatures.
Nickel-iron alloy plating, commercialized in the early 1970s, may prove to be the first step towards the development of a method for electrodepositing stainless steel. The codeposition of as little as 10 percent chromium in an alloy containing little or no carbon would be ample for providing good corrosion resistance and obviating the costly two-, three- or four-step processes used today for plating a composite of nickel and chromium. The search for an effective process is being actively pursued in Japan, but the potential high value of a workable process justifies an expanded effort.
Rapid, turbulent flow of the plating solution, important for achieving fast-rate plating, promotes efficient deposition of chromium and should be effective for assisting the deposition of chromium alloys. In the case of unalloyed chromium, efficiency can be tripled or even quadrupled by adopting a high rate of solution flow. Thus, the development of a practical method for codepositing chromium with nickel, cobalt and iron may be imminent.
The dispersion-hardened alloys obtained by codepositing small particles of hard oxides or carbides in a matrix of chromium, nickel or other metal represent another area for future opportunists looking for better wear-resistant coatings. Technologists have only begun to exploit the advantages of this kind of coating, which offers prospects for greatly extended wear life. There are many potential applications for such coatings, which can be used profitably for supplanting chromium and electroless nickel on steel and anodic coatings on aluminum. New uses are inevitable.
Electroforming - Originally employed chiefly for producing art forms, electroforming has been growing slowly from infancy to puberty and now is recognized internationally as a cost-effective method of fabricating thin-wall complex shapes. Fast plating techniques will help to promote further growth, especially if a method is perfected for forming true, dispersion-strengthened materials consisting of ultrafine inert particles cohesively bonded to the electrodeposited matrix. Materials with a tensile strength as high as 200 kg/mm2 and a modulus of elasticity as high as 31,500 kg/mm2 have been produced experimentally. This combination of properties is attractive for a variety of electroforming applications. Improvement in the cohesive bond between the particles and the matrix and the codeposition of a small amount of manganese for improving the properties of nickel at high temperatures can be expected. When achieved, the potential for electroforming will be further enhanced.
Smoothing/deburring - Methods of surface smoothing and deburring have improved significantly in the past 20 years and will continue to evolve in the future. Slurry polishing and vibratory processes that greatly accelerate the smoothing process are matters of record, as are chemical polishing processes. The synergistic effect documented a few years ago of combining mechanical energy in a vibratory mode with chemical action suggests that other somewhat similar combinations may provide even faster processes that improve machine productivity, conserve energy and reduce cost. Opportunities exist here for engineers with both a mechanical aptitude and a chemical bent.
Nonelectrolytic deposition - Among the several expanding nonelectrolytic methods of applying coatings, mechanical impact plating has been growing steadily. Using metal powder, surface active agents, impacting media such as glass beads, and mechanical energy, this process deposits compact coatings of soft metals such as cadmium, tin and zinc. A process for plating mixtures of cadmium and zinc and a two-layer zinc-tin system recently were developed for steel fasteners and other applications. Future growth of this impacting process undoubtedly would be enhanced if a method were perfected for accommodating the deposition of aluminum or aluminum alloys. Such coatings would be useful for many diverse applications requiring lubricity and corrosion resistance. Here is another opportunity awaiting the acumen and dedication of an opportunist.
Pollution control - During the past few years, a large proportion of the technical reports on plating and surface finishing has dealt with pollution control. These reports reflect the opportunistic developments of hundreds of technologists. As a result, better methods for conserving materials and reducing the cost of pollution control now are available. Improvements in membranes for reverse osmosis and electrodialysis, in vacuum evaporation and ion exchange, and in electrolytic recovery have been noteworthy, to mention just some of these developments. Even so, the search for still better, more effective methods of reducing overall costs and minimizing pollution is still going on. Liquid ion exchange, a promising technique for recovering metals from rinsewater, appears to have been neglected until just recently. The removal of metal compounds with selective organic solvents and their subsequent transfer to concentrated aqueous solutions offer promise for unique systems that may prove to be more cost effective than the currently available methods. The search for suitable organic solvents justifies more attention from opportunists with interest in pollution control.
In any case, opportunism, which has been responsible for the growth of our nation, must be encouraged to sustain future progress. There are many mysteries to be solved. All we need are the detectives - the opportunists!






.jpg;maxWidth=600)













