Preventing Solvent Pop
Preventing solvent pop on an industrial paint line...
#curing
Our Research and Development Center at Akzo Nobel Coatings, Inc., recently ran an experimental design for a client who manufactures and paints heavy-duty equipment. We needed to determine the optimum factors for increasing paint film thickness without solvent popping occurring. Our client wanted us to make sure that painted panels exited the ovens hard and cured, without any film blistering or softness.
Using design of experiments (DOE), Akzo performed a five-factor, two-level, 16-experiment design. We evaluated the five factors and their 10, two-factor interactions by taking an average of the eight high and low responses for each of the 15 factors and their interactions. The factors and levels are shown in Table I.
Featured Content
The experimental design and analysis work was simplified by using DOE software named Design-Ease® (Stat-Ease, Inc., Minneapolis, Minnesota). The software allowed us to consider all factors simultaneously by revealing how interconnected factors responded over the range of values.
| TABLE I—These Factors and Levels Simulate the Client's Industrial Production Line | ||
| FACTOR | LOW LEVEL (-) | HIGH LEVEL (+) |
| A: Convection oven temperature (F) | 100 | 200 |
| B: Infrared oven temperature (F) | 125 | 300 |
| C: Accelerator Per drum (ml) | 850 | 1000 |
| D: Activator type: Polyisocyanates | A | B |
| E: Convection oven air flow | Blocked | Open |
The designed experiment simulated our customer's paint curing ovens so we could determine the best settings for reducing solvent pop. The solvent pop response was measured as the film thickness at which popping began. The desired outcome was to have high, tack-free film build without any popping. In particular, we needed to pay careful attention to the grueling factors encountered during the hottest part of summer. This is the time of year when solvent pop is most common, especially in a facility that is not climate controlled—such as our client's. Popping occurs more frequently in hotter environments as film thickness increases. Therefore, it is important to carefully control the ovens to avoid solvent popping. The film coatings must endure extremely harsh conditions, year after year, in all types of soil, weather and temperatures. The coating's function is to protect heavy-duty specialty outdoor equipment.
Manipulating factors to attain an ideal response. Our objective was to vary and test important factors for solvent pop. We began by preparing eight, one-inch by four-inch test panels, then ran the panels through our laboratory simulation paint line. The simulated procedure was as follows:
A.Apply with airless sprayer 1.0 -1.5 dry mils of primer on all eight panels.
B.Flash for six minutes.
C.Apply two coats of paint onto the eight panels.
D.Remove one panel.
E.Apply one coat of paint onto the remaining panels.
F.Continue removing one panel and applying a coat of paint to the remaining panels until they contain between two coats (low film thickness) and nine coats (high film thickness) of topcoat.
G.Flash for six minutes.
H.Cure in convection oven for 12 minutes.
I.Cure in infrared oven for 12 minutes.
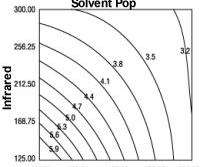
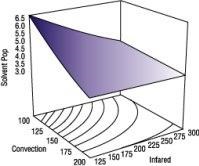
The DOE software's analysis showed that the most significant factors causing solvent pop were convection and infrared oven temperatures. Although there was significant interaction between both oven types, of the two, the convection oven was a bigger factor than the infrared. Refer to Figures 1 and 2.
By combining the correct oven temperatures, along with proper accelerator levels and activator types, we were able to consistently minimize solvent pop with acceptable film hardness and cure. The settings we obtained from the DOE software to minimize solvent pop are:
1.Low convection heat (Factor A-).
2.Low infrared heat (Factor B-).
3.High catalyst or accelerator.
4.Activator B.
By simulating our client's curing oven factors in a laboratory DOE, we optimized the settings that reduce solvent pop. Our client can now expect fewer application problems and higher production efficiencies.
RELATED CONTENT
-
Infrared Curing: Evaluating the characteristics of IR energy
Infrared (IR) energy can be used as a source of heat to cure a variety of industrial coatings. Such infrared curing applies energy to the coated part surface by direct transmission from an IR emitter, which can provide source temperatures of anywhere from 500 to 4,200°F
-
Understanding Infrared Curing
Infrared cure is gaining increased attention from coaters as a result of shorter cure cycles and the possibility of smaller floor space requirements when compared to convection oven curing.
-
Reducing Powder Coating Waste
Coating application is often overlooked as a potential money saver, but recognizing opportunities within this area is crucial to an efficient operation.