Reinventing Chrome Coatings
Low-temperature arc vapor deposition can provide an alternative to hexavalent chromium plating.
#vacuum-vapor
Hexavalent chromium plating has long been a favorite surface finish due to its hardness, corrosion resistance and shiny appearance. However, hexavalent chromium is a known carcinogen and industrial exposure levels are mandated by U.S. law to be reduced by as much as 50 times by January 2006. It’s obvious that an environmentally friendly alternative for this popular finish is needed.
One possibility is low-temperature arc vapor deposition (LTAVD). LTAVD occurs in a controlled vacuum environment and is a type of physical vapor deposition (PVD). Able to operate over a range of temperatures, LTAVD deposits adherent and dense metallic and ceramic coatings on traditional metal substrates as well as heat-sensitive materials such as plastics and zinc die-casting alloys. It results in a very durable coating that can also be used to make low-cost materials look like more expensive metals such as stainless steel, nickel, bronze or other materials.
Featured Content
Stack Engineering
Conventional processing of parts for cosmetic coating begins with polishing, brushing or grit blasting the part to be coated, followed by a layer of nickel/chrome plating. High-end products often add a PVD metallic or ceramic coating that simulates the appearance of various metallic finishes. These coatings can be deposited directly onto the chrome-plated surface or onto a properly prepared substrate.
Hexavalent chromium plating historically has been more cost-effective than the alternative PVD chrome coating. However, the U.S. Department of Labor’s Occupational Safety and Health Administration (OSHA) has proposed a new standard for occupational exposure to hexavalent chrome that would lower the permissible exposure level from 52 µg/m3 to 1 µg/m3 over an eight-hour time period.
OSHA’s new restrictions would become effective in January, 2006. Many in the surface finish industry feel that the cost to comply would be so prohibitive that new technologies must be employed or most chrome plating operations would move off-shore or go out of business. Therefore, interest in the PVD chrome coating process has gained significant momentum due to general uncertainties about the future for hexavalent chromium-based plating chemistry and its increasing costs.
Popular PVD coatings incorporate zirconium, chromium, titanium, alloys, nitrides, carbides and oxides. The success of these coatings is based on their ability to achieve the desired appearance while providing superior durability. All layers of the coating stack must be designed to meet part requirements for resistance to wear and corrosion as well as appearance.
The PVD process supports creation of very sophisticated coating stacks. In fact, most standard PVD coating stacks have, but are not limited to, up to five distinct layers.
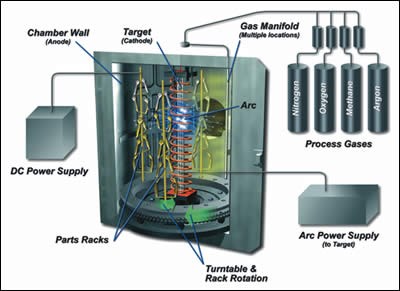
LTAVD processing is accomplished in a plasma created from a mixture of evaporated metal, metal ions and gases such as argon, nitrogen, oxygen and methane. A number of technologies for metal evaporation and ionization are commercially available, but technology using a centrally mounted target (cathode) has proved superior for a number of reasons, including: high throughput, high- and low-temperature processing, and optimal uniformity and adherence of its coatings.
Testing, Testing
In one experiment conducted by our company, ASTM A36 carbon steel coupons were plated in a conventional electrolytic plating line. Three coating stacks, consisting of varying nickel base coats and a top-coat of chrome plated from a hexavalent chrome bath, were completed by an outside vendor. A total of 140, 10 × 10 cm coupons were plated. Two-thirds of the chrome-plated coupons were stripped in an anodic alkaline solution (chrome was initially applied on all parts to prevent oxidation before the experiment) and then re-coated in a VT1500 LTAVD chamber equipped with a centrally mounted chromium target applying chromium and chromium reacted with nitrogen to form chromium nitride (CrN).
Copper-accelerated salt spray (CASS) testing was performed according to ASTM B 368, and results were evaluated according to ASTM B 537-70. Analysis included elemental profiling via glow discharge spectroscopy (GDS), color characterization, abrasion resistance testing, and hardness and modulus profiling.
| Table I: Experiment matrix of coating stacks | |||
| Underlying Plating |
Hex Cr | PVD Cr | PVD CrN |
| Bright Nickel |
x | xx | xx |
| Duplex Nickel | x | xx | xx |
| Micro-porous Nickel | x | xx | xx |
| Note: x = original Hex Cr plating; xx = coating applied via PVD | |||
The plating stack was targeted at a total nickel thickness of 25 microns with a chromium top coat 0.25 microns thick. This plating stack is appropriate for service condition #3 (severe) as specified in ASTM B 456. The coating stacks are summarized in Table I.
GDS elemental analysis of a typical duplex plating stack showed the top chrome layer (250 nm) followed by high sulfur content in the upper half of the coating stack corresponding to a bright nickel layer. The deeper portion of the nickel layer shows carbon and sulfur levels below 0.1 atomic percent—analysis characteristic of a matte nickel.
Two-thirds of the samples were stripped of hexavalent chromium and recoated with PVD chromium (Cr), or PVD chromium and chromium nitride (CrN). One third were coated with just Cr, while the other third were coated with a Cr/CrN/Cr “sandwich”. The outermost Cr layer determines color, the CrN layer provides hardness, and the innermost Cr layer optimizes adhesion to the substrate.
Corrosion Performance
According to ASTM B 456, combinations of nickel thickness and thin chrome top layers improve corrosion resistance as a function of increasing nickel thickness. Corrosion resistance can be improved further by one or more of the following:
- Start with a noble nickel layer (semi-bright nickel) followed by a less-noble nickel (bright nickel) in combination, called duplex nickel, ending up with a thin chrome top layer.
- Start with a bright nickel layer followed by a thin, microporous nickel layer, ending up with a thin, microporous
chrome top layer. - Increase the number of cracks in any given chrome layer (micro-cracked).
A chrome layer produced via a hexavalent chrome process also will have somewhat improved corrosion resistance on uncoated surfaces due to a chromate conversion coating effect.
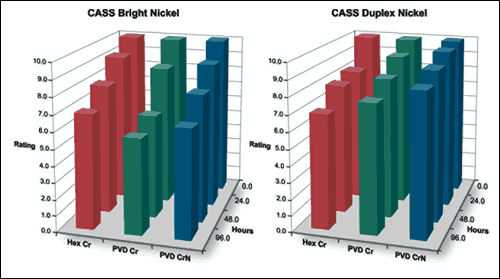
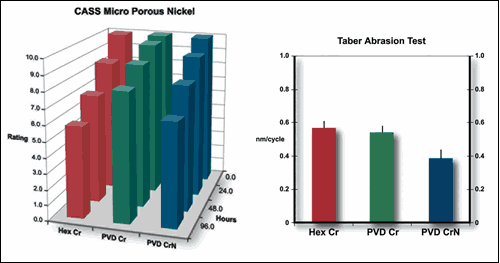
Given a pass criteria of rating 9 (< 0.1% attacked surface area), most of the samples passed 24-hour CASS testing. The samples showed corrosion as a result of pinhole defects bleeding red rust from the steel/nickel interface. Other types, like nickel corrosion, were not observed.
Duplex and microporous nickel coatings show a beneficial effect on small-diameter pinhole corrosion and move the corrosion site away from the steel/nickel interface. Therefore, the 24-hour rating is effectively a measure of large pinhole density.
If the bar is lowered to a rating of 8 (0.25% attacked surface), the superior performance of the duplex and microporous nickel becomes evident. In all instances, the PVD Cr and PVD CrN coatings show CASS performance comparable to hexavalent chromium if not better.
Color Results
As measured by customer preference, hexavalent chromium has a very desirable color that is often described as slightly blue. We evaluated the colors of our samples in a L*a*b color space using a Minolta CM 508d spectrophotometer, D65 light source, and a 10o observation angle. Color difference (ΔE) between hexavalent chromium and PVD chromium averaged about 1.4. Generally, differences may not be apparent to a trained observer until E exceeds 2.
Hardness and Elasticity
The hardness of a coating is determined by the penetration depth of a diamond stylus. As a rule of thumb, only measurements from the upper 5-10% of layer thickness represent layer hardness and modulus; the remainder represents a weighted average of two layers.
Our Vickers hardness measurements show PVD chromium hardness of 800-1,400 Hv and PVD CrN hardness up to 2,200 Hv. Hexavalent chromium indicates hardness of 600 to 700 Hv. Bright nickel is about 800 Hv.
Abrasion Resistance
Even though very high hardness and modulus values can be found in literature for a given coating, characteristics such as wear and friction performance under specified load conditions more often determine its applicability. These attributes are dictated by the characteristics of a combination of the top coating, its supporting layers and the substrate.
The Taber abrasion test was developed to characterize wear performance of organic coatings. We adopted it to visualize wear performance of hexavalent chromium, PVD chromium, and PVD CrN surfaces.
In Taber abrasion tests using a CS10 wheel and 1-kg load, hexavalent and PVD chromium appeared to have equivalent abrasion rates. Wear on PVD CrN is about 30% better, and this translates to a longer service life.
The question of PVD Cr as a viable alternative to conventional Hex Cr plating will of course be decided by the market, based on continued financial and regulatory pressure on hexavalent chrome. However, this investigation shows that PVD Cr is functionally equivalent, if not better, while offering many more decorative alternatives.
RELATED CONTENT
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.
-
A Chromium Plating Overview
An overview of decorative and hard chromium electroplating processes.
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.