Electroless nickel coatings are widely used because of their uniform thickness, corrosion resistance, hardness, wear resistance and magnetic response. Some of these properties are affected by internal stress in the coating, however. Very often, internal stress can be the most significant criterion for determining the suitability of an EN process for a specific application. Here, we will discuss the factors that impact this stress.
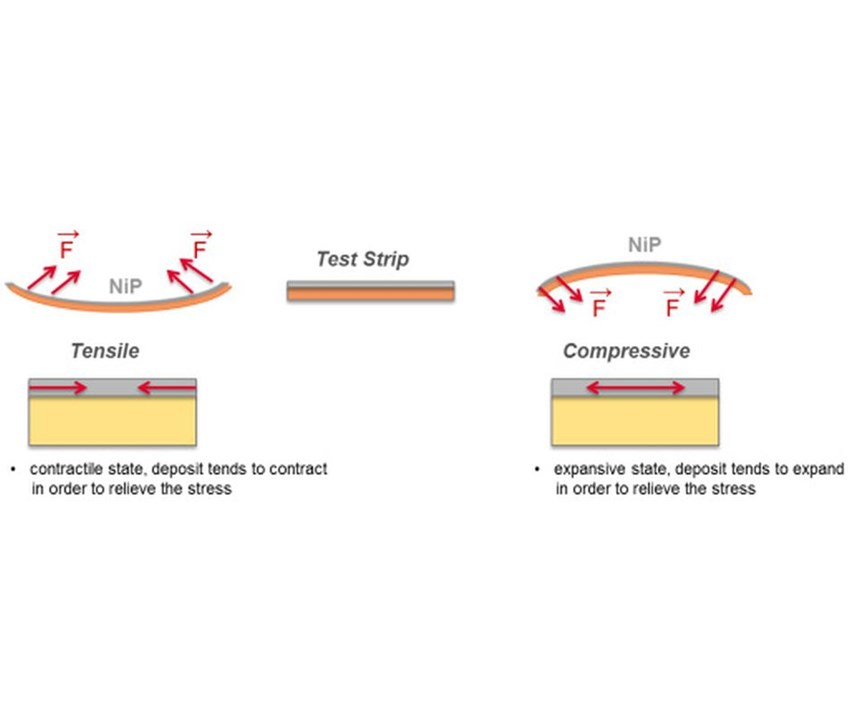
Internal stress consists of an intrinsic component resulting from the composition and age level of the plating bath, and a thermal component produced by the difference in the thermal expansion coefficient between the EN coating and the substrate. Aluminum, for example, registers strong compressive stress values due to the high shrinkage the occurs when it cools from the working temperature of the plating process to room temperature.1 Internal stress can be of a tensile or compressive nature. Under tensile stress, the coating tends to reduce in volume and shrink (contraction state); under compressive stress, it tends to expand by increasing in volume (expansive state).
Featured Content
Intrinsic stress is produced by any deviation from the perfect crystalline order of a solid.2 Deviations on the atomic scale consist of lattice mismatch due to the difference between the substrate and the deposit’s crystalline structure, or due to inclusion of foreign atoms (interstitials) or vacancies. At the mesoscopic level, dislocations and grain boundaries are the most usual deviations. At the microscopic level, grain boundaries of coarse crystalline materials can occur. At the macroscopic level, all mechanical forces applied to a piece and differences in the coefficient of thermal expansion can create stress. Vectorial addition of all forces, from atomic to macroscopic, produces the measured stress of a coating.
Deflection Measurement
The deflection method is the most widely used technique for measuring residual stress in a coating. This method is based on measuring the amount of bending in a sample due to deposition of the coating. The formula for calculating residual stress was first derived by G.G. Stoney3 in 1909, but more complex formulas have since been derived that provide more accurate stress evaluation.4 One is based on the concept that the sum of resulted forces at the interface of substrate and coating leads to residual stress in the coating, and this can bend the test strip either concave, in the case of tensile stress, or convex, in case of compressive stress (see Figure 1).
The most used devices for stress measurements using this principle are:
- Bent strip method, in which a test strip deflects due to the deposit stress. The strip is split into two legs with single-sided lacquer insulation to prevent stress compensation. The deflection is read by placing the test piece over a scale that is quantified by increments. Stress then can be calculated using a simplified version of Stoney’s formula.
- Use of a Brenner-Senderoff spiral contractometer, in which the test piece is a spiral with one end free to move. Movement can be registered in degrees over a dial, and the resulting stress can be calculated. This method requires a relatively large plating bath volume, and the used spiral must be stripped after each measurement.
The bent strip method is the most suitable technique for conditions typical to a production plating environment, allowing quick determination of stress not only in EN coatings, but in ceramic and polymer films as well.
X-ray diffraction is the only method that enables the measurement of internal stress directly on the actual plated part, however, it can be used only on crystalline deposits. In the case of nickel-phosphorus alloys, the dominant amorphous structure of high- and mid-phosphorus EN coating leads to peak broadening, which makes the evaluation impossible.
Factors Impacting Stress
A series of experiments were conducted using the deflection method on stress strips. The electroless nickel processes used were within the usual industrial concentration range of nickel (5–6 g/l) and sodium hypophosphite (20–30 g/l), and compliant with End of Life Vehicle (ELV) 2000/53/EC.
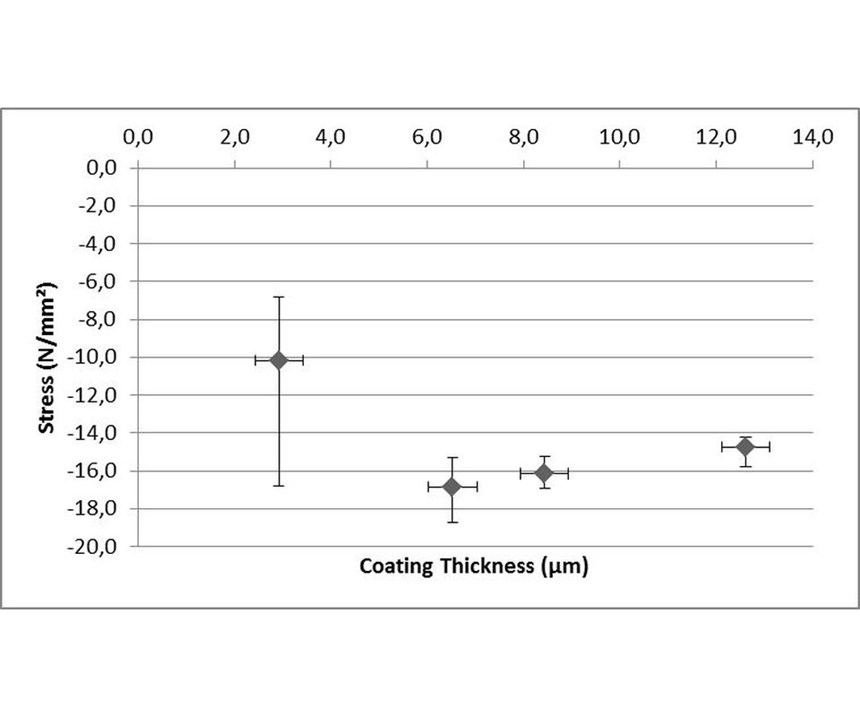
Coating thickness: Experiments were conducted in-situ in a 65-liter tank with an ELV-compliant high-phosphorous EN process. For each thickness, three test strips were coated at the same time under the same conditions: pH of 4.9 and temperature of 87°C. Bath load was 0.5 dm²/l with steel. Plotting of stress versus coating thickness shows that the stress values became essentially constant at coating thicknesses above 8 microns. Measurements on a coating thickness of less than 8 micons have a higher uncertainty and lower reliability. In the literature, 12 microns is considered the minimum coating thickness for reaching reliable stress values.6
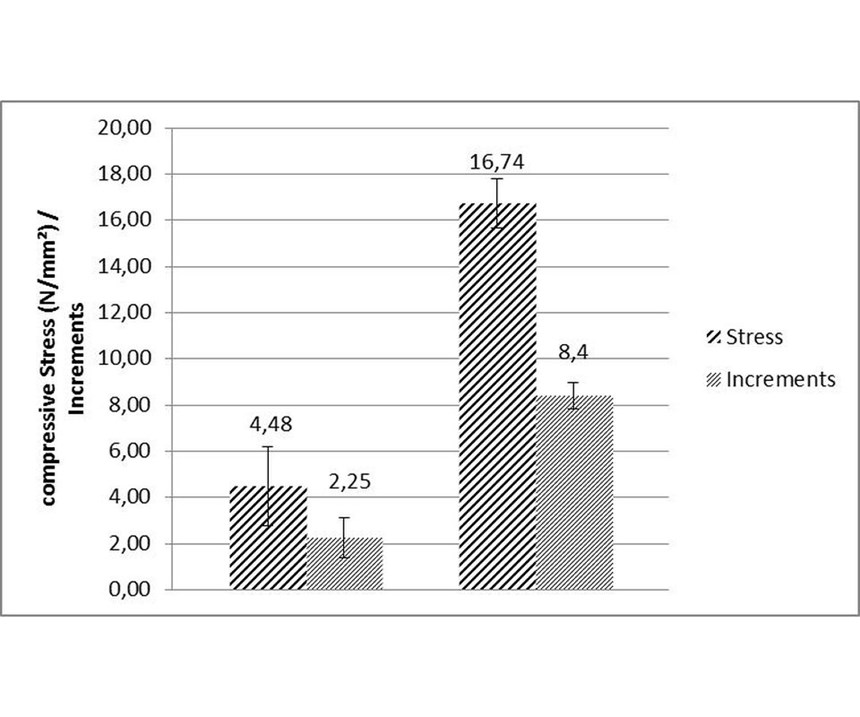
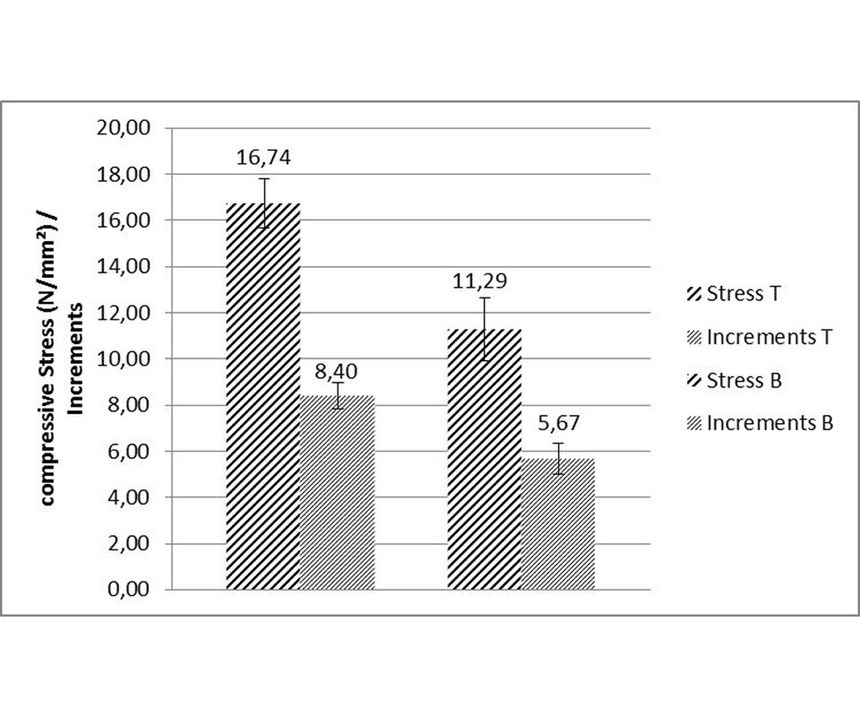
Bath volume: Tests were run with two different ELV-compliant high-phosphorous EN processes with compressive stress. Graphical evaluation was in absolute values for a better visualization. For the first set of runs, the stress values were slightly compressive (see Figure 3, left side). The second process produced coatings with higher compressive stress (see Figure 3, right side). Each set of runs was conducted with 10 strips coated at same time. Reproducibility was limited to ca. ±1 increment, corresponding to an uncertainty of ±2 N/mm² absolute value. Consequently, the relative uncertainty decreases with increasing deposit stress. Following experiments were conducted in a 1-liter beaker using the same process solution of the strong compressive, high-phosphorus EN as in the tank. Both were operated at a pH of 4.9 and 87°C. Data evaluation showed a standard deviation of ±1.5 N/mm² and lower stress values than in the tank (see Figure 4).
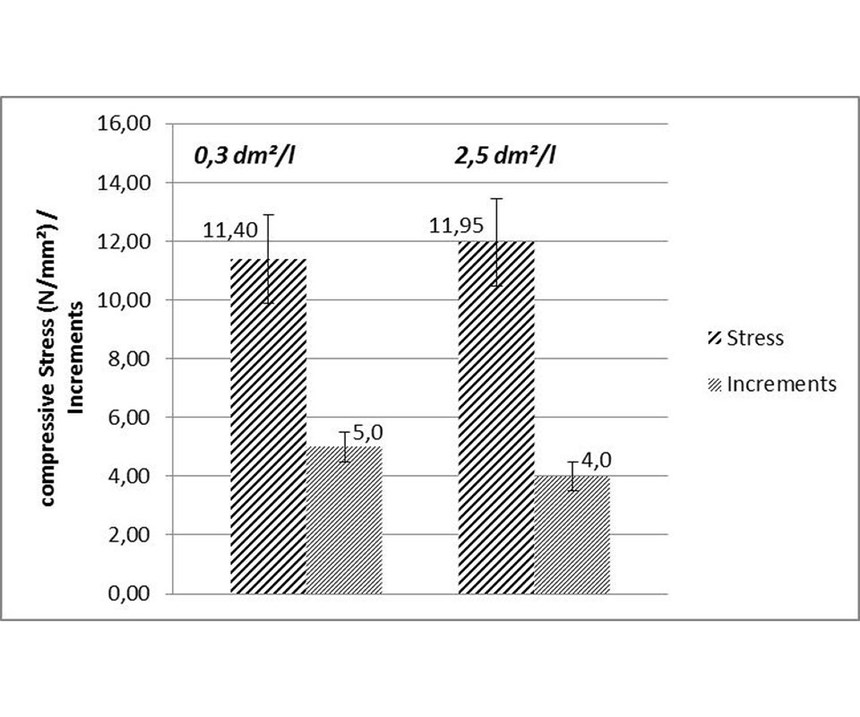
Bath loading: Experiments were done in a 1-liter beaker with an ELV-compliant mid-phosphorus EN process at a pH 5.0 and temperature of 88°C, using steel panels with 0.3 and 2.5 dm² and three stress strips for each beaker. From Figure 5, it is obvious that bath loading had no significant influence on stress; the difference was within the range of the standard deviation of ±1.5 N/mm² for measurements in a 1-liter beaker.
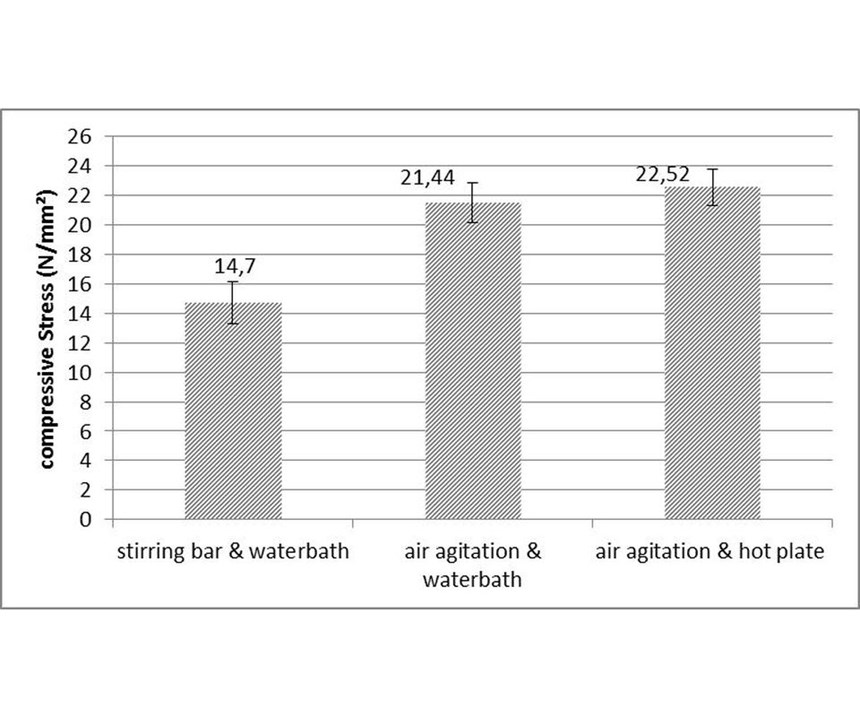
Bath convection and heat distribution: Experiments were conducted by combining water bath and a hot plate as heating sources, with a stirring bar and air agitation as bath convection. A high-phosphorus, non-metal, stabilized EN process was used at a pH of 4.7 and temperature of 88°C with compressive stress. The experiment setup is depicted in Figure 6. Figure 7 shows that air agitation promotes stress formation, and this can explain the results obtained by comparing stress values in the tank with those in the 1-liter beaker, since air agitation was used in the tank and a stirring bar in the beaker. Heat distribution using a water bath or hot plate had no influence on stress formation.
Temperature and pH: It is well-known that pH and temperature are the parameters that have the biggest impact during EN operation. There is a synergistic effect between these two parameters.
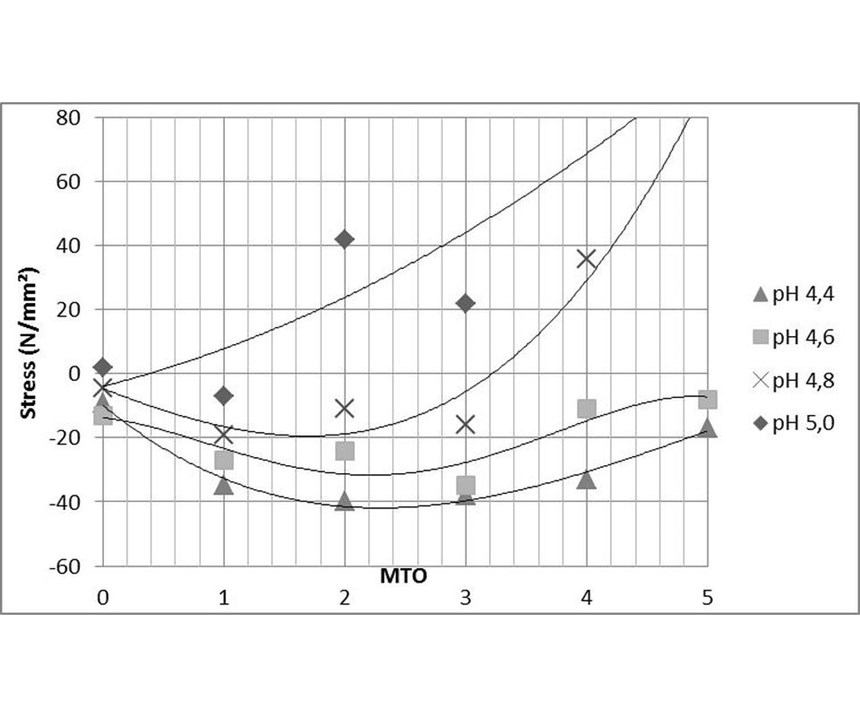
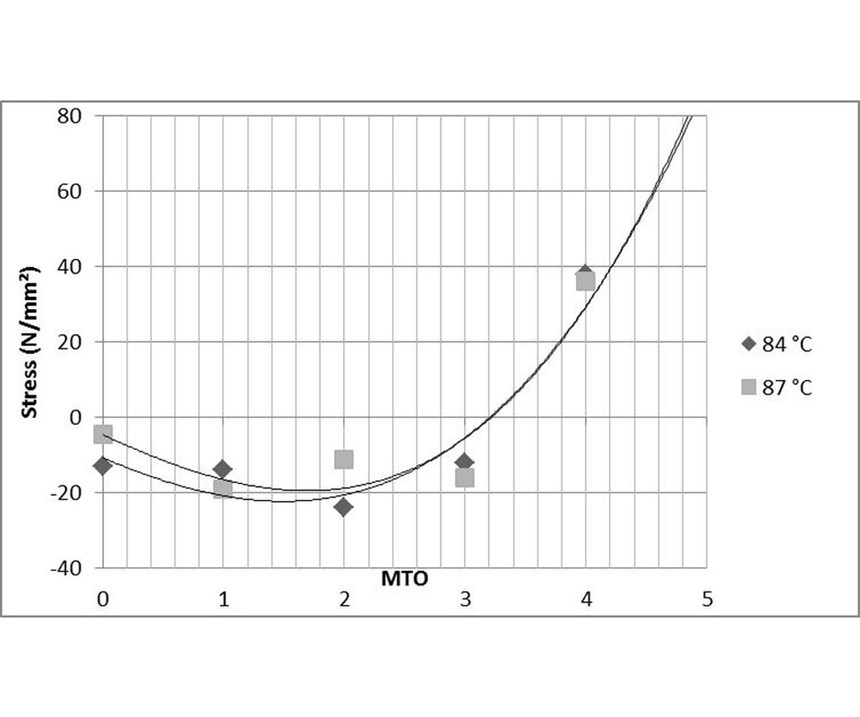
Experiments were done with an ELV-compliant high-phosphorous EN process operated in 60-liter tank. Each MTO, 1-liter samples were taken out for investigation. The pH was adjusted from 4.4 to 5.0, while the temperature was kept constant at 87°C for all samples (see Figure 8). At pH below 4.5, stress values remained in the compressive range until 5 MTO (25 g of nickel deposited). At pH of 4.8, a small shift towards tensile can be observed. Measurements done at pH of 4.6 showed a transition point from compressive to tensile at around 3 MTO (15 g of nickel deposited). At pH of 5.0, the transition from compressive to tensile takes place between 0 and 1 MTO (5 g of nickel deposited).
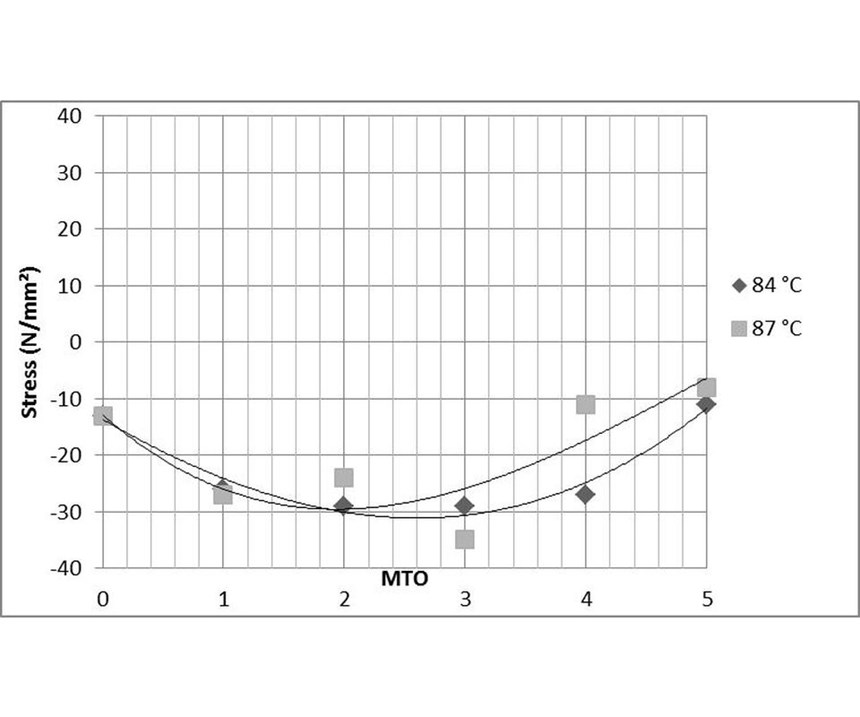
Comparable experiments were conducted at temperatures of 84°C and 87°C, and two sets of data were generated at pH of 4.6 and 4.8 (see Figures 9 and 10). The process bath temperature was found to have far less influence on stress than the pH of the EN solution.
Similar results were reported by Z. Chen et al.7 when stress measurements were made on artificially aged EN solution at different pH values. The team concluded that bath ageing by deliberate salt addition and high pH values have negative influence on stress, while stress remains constant at lower pH despite high salt concentration.
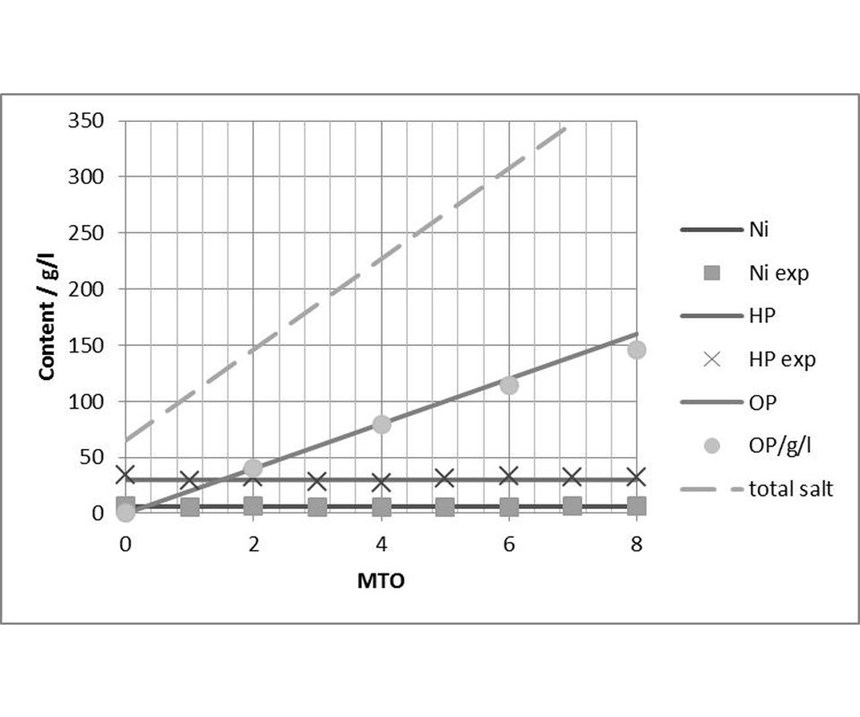
Bath age: Figure 11 shows the general trend of the most important components of an EN process. During throughput, the concentration of nickel (Ni) and of the reduction agent (HP) were maintained constant. The amount of generated sodium orthophospite (OP) increased with about 0.3 mol per MTO (6 g of deposited nickel). Total salt concentration and bath density rose steeply with bath age. the first process performance issues usually start at a bath density of around 1.25 g/cm³.
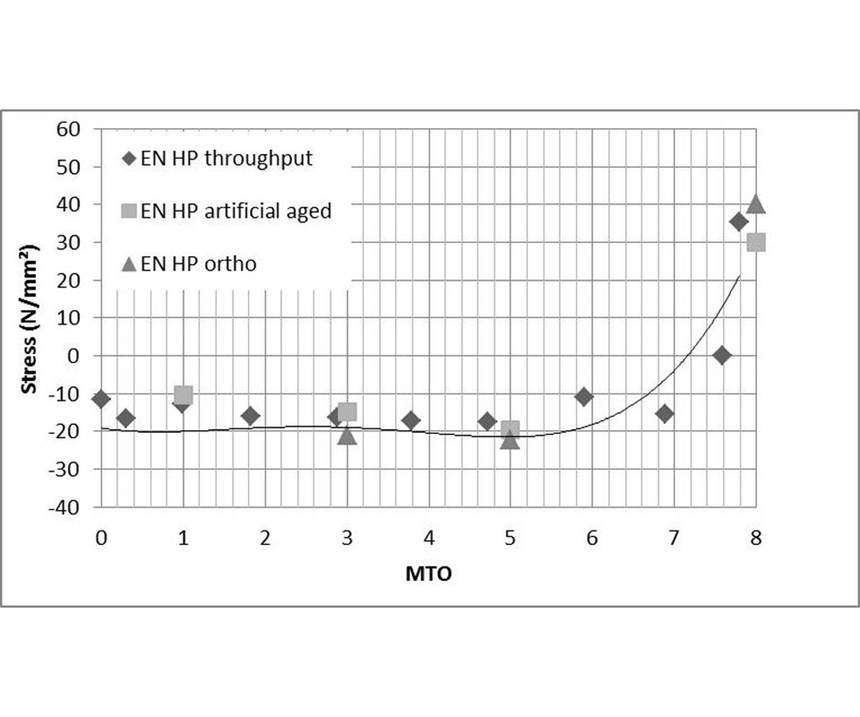
In order to establish the influence of the most important generated byproducts, stress measurements were conducted on artificially aged EN solutions. Figure 12 shows the comparison between stress values from the normal throughput, stress values after artificially ageing with the most important byproducts of the EN reaction and stress values obtained after adding sodium phosphite. Artificial ageing simulated very well the real bath ageing. Stress values after artificial ageing matched very well with stress values of the real throughput under the same pH and temperature conditions. Since stress values obtained after adding only sodium phosphite were similar to those of the real throughput and of the artificially aged by adding all byproducts, it is obvious that the orthophosphite level in the EN solution had a relevant impact on the stress. In the real throughput, orthophosphite is the byproduct with the biggest impact on solution density, on the process performance and on the stress in the coating. Coatings deposited from a bath containing excessive orthophosphite concentrations typically exhibit high tensile stress, increased porosity and reduced corrosion resistance.
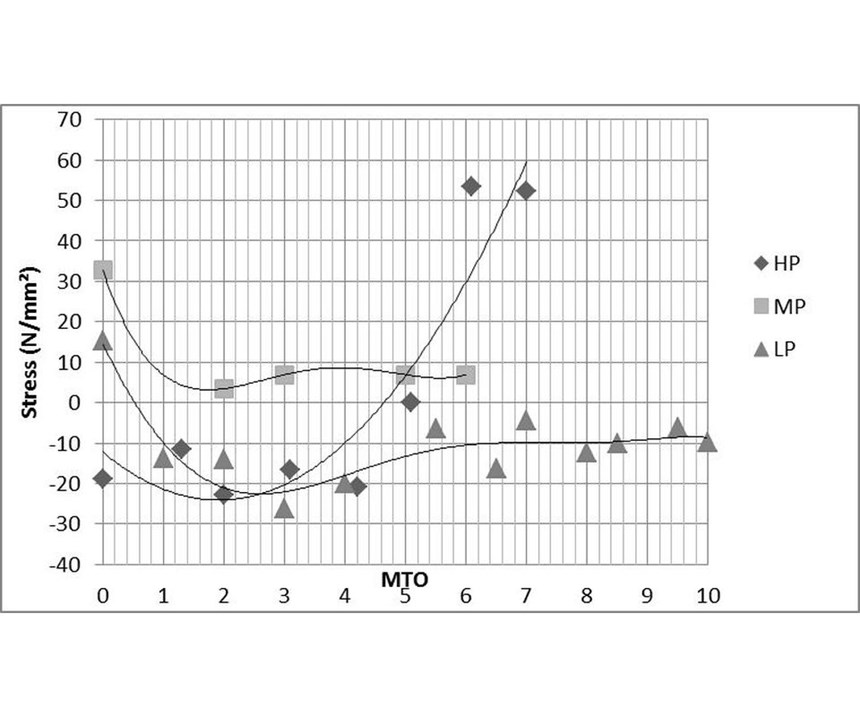
Phosphorous content: It is known from literature8 that deposits with high phosphorous incorporation are compressively stressed when plated from a new bath, but the stress becomes progressively more tensile as the bath ages. Deposits with low phosphorous content are compressively stressed from new or aged baths, and those with medium phosphorous content are usually in a state of tensile stress. Figure 13 shows the stress trend over bath life for high-, mid- and low-phosphorous processes. Nevertheless, there are mid-phosphorus ELV-compliant processes that, due to their chemical formulation, remain compressive over bath life so that a well-balanced process composition can counteract the strong influence of the phosphorous content.
More extensive studies need to be done regarding the influence of contamination or special additives, since this is specific for each EN process. Generally, it is well-known that usual contaminants such as dissolved foreign metals, organic compounds, anions and silicon compounds can adversely affect coating properties including internal stress.
Stress Shift with Storage Time
The deflection of stress strips is known to change with storage time under ambient conditions. Recrystallisation processes, superficial oxide layer formation or effusion of codeposited hydrogen are possible reasons for this behavior.
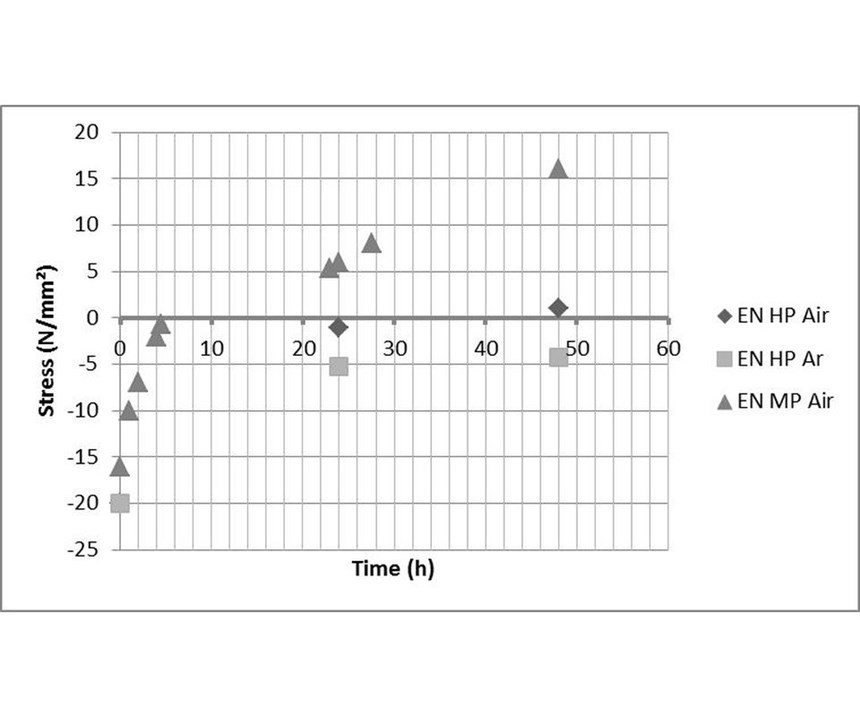
For example, stress strips coated with electroless nickel were stored over a period of 48 hours in air and in an argon atmosphere. From Figure 14, it is obvious that stress shifts with time to the tensile range, both under air and under argon. Considering the time scale and the ambient temperature, recrystallization is not a feasible explanation for the deflection change. Oxide formation was excluded by a benchmark test in an inert gas atmosphere. Thus, the release of incorporated hydrogen from the nickel-phosphorus layer is the most likely explanation for the time dependence. This may lead to a volume contraction and shifts the stress towards the tensile direction.
Conclusions
Internal stress in EN coatings can be influenced by many factors and parameters. Some of them can be changed in order to favorably impact stress, others cannot. A minimum coating thickness and comparable process bath agitation are required to obtain reliable stress measurements.
These tests indicated that the factors with the biggest impact on stress are the age of the EN solution and the deposition rate. Stress is much more influenced by the pH of the plating solution than by bath temperature. Increasing pH during EN operation in order to reach a higher deposition rate leads not only to a decrease in phosphorous content, which affects coating properties, but also to a much earlier transition point from the compressive to tensile stress range. The phosphorus incorporation also has a significant influence on the deposit stress. Finally, it is important to note that the stress properties of EN deposits change within short storage times.
References
1K. Parker, American Society for Testing and Materials (1987) 111
2K. Romankiewicz, K. Schmid, M. Metzner, Galvanotechnik 10 (2007) 2366
3G.G. Stoney, Proc. R. Soc. (London), vol. 82, 1909
4T. Sanderson, Surface and Coatings Technology 202 (2008) 1493
5P. J. Withers, H. K. D. H. Bhadeshia, Materials Science and Technology 17 (2001) 355
6E. Crotty, N. Micyus, Journal of Applied Surface Finishing 2008
7Z. Chen et al. Surface and Coatings Technology 167 (2003) 170
8R. Parkinson, Nickel Development Institute, Properties and Applications of Electroless Nickel
Dr. Iulia Bejan is a scientist with Atotech Deutschland GmbH in Berlin, Germany. She can be reached at iulia.bejan@atotech.com.
RELATED CONTENT
-
Blackening of Ferrous Metals
The reasons for installing an in-house cold blackening system are many and varied.
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Choosing and Troubleshooting Copper Electroplating Processes
Learn more on this inexpensive and highly efficient process.