The Formulation of Electroless Nickel-Phosphorus Plating Baths
Originally published as K. Parker, Plating and Surface Finishing, 74 (2), 60-65 (1987), this paper was awarded the 1988 AESF Gold Medal for Best Paper published in Plating and Surface Finishing in 1987. The functions of various bath components are discussed in detail. Organic acid complexants, buffers and stabilizers were added to a lactic acid bath to determine their effects on plating rate, bath stability and deposit resistance to nitric acid.
Featured Content
by
Konrad Parker
Editor’s Note: Originally published as K. Parker, Plating and Surface Finishing, 74 (2), 60-65 (1987), this paper was awarded the 1988 AESF Gold Medal for Best Paper published in Plating and Surface Finishing in 1987. A printable version of this paper is available by clicking HERE.
ABSTRACT
The functions of various bath components are discussed in detail. Organic acid complexants, buffers and stabilizers were added to a lactic acid bath to determine their effects on plating rate, bath stability and deposit resistance to nitric acid.
Since its accidental discovery in 1944 by Brenner and Riddel,1 electroless, or autocatalytic, nickel plating has grown from a laboratory curiosity to a $100 million industry. The first alkaline ammonia baths contained nickel chloride, sodium citrate and/or ammonium chloride, and sodium hypophosphite as the reducing agent. The early acid baths2 were formulated with either hydroxyacetate (glycolate) or citrate as the nickel complexant.
The Kanigen* process,1 developed in the 1950s, contained lactic acid4 as the complexing agent, propionic acid as the exaltant,5 and a trace of lead6 as a stabilizer. The bath was filtered and heated by continuous circulation using external steam heat exchangers. Depleted chemicals were replenished in a separate tank after cooling the bath to 80°C. The process gained widespread acceptance due to its fast plating rate, good solution stability, and relatively long bath life.
Proprietary electroless nickel baths, which have been marketed in concentrated form for about 20 years, contain combinations of complexing agents, buffers, and stabilizers. The baths are the result of much empirical research and development. The technical data sheets of suppliers disclose only the nickel and hypophosphite content. Few new formulations have been published; Gutzeit's 1959 articles7 on bath chemistry are still the best source of information. Numerous patents claiming improvements in baths or deposits have been granted. Most of these disclose various stabilizers or additives.
Electroless nickel plating solutions based on boron reducing agents such as sodium borohydride and dimethyl-amine borane have also found select applications that will not be covered here.
The mechanism and kinetics of the autocatalytic plating process have been discussed8,9 and are still under investigation. The reduction of nickel ions by hypophosphite involves several competing oxidation-reduction reactions in which phosphorus is always codeposited:
catalyst
Ni+4 + H2PO2- + H2O ⇒ Ni + H2PO3- + 2H+ (1)
catalyst
H2PO2- + H ⇒ P + OH- + H2O (2)
catalyst
H2PO2- + H2O ⇒ H2PO3- + H2 (3)
Reaction 1 is favored by high pH, and Reactions 2 and 3 by low pH. The plating rate therefore increases as the pH is elevated. Thus, to obtain a uniform rate and a deposit with a consistent phosphorus content, the pH must be controlled within narrow limits.
The plating rate also increases exponentially with solution temperature. Most acid electroless nickel baths operate best at 85 to 90°C. To maintain a consistent deposition rate, the bath temperature should be as uniform as possible throughout the plating tank. This is accomplished by agitation. Bath loading is another critical factor. When at operating temperature, the bath should always be loaded with at least 50 cm2/L (0.2 ft2/gal) of work surface to avoid decomposition, plateout and edge misplating when high solution agitation is employed.
The plating reactions produce sodium orthophosphite, hydrogen ions and hydrogen gas as byproducts. These create problems, which will be discussed later. Also, about 16 g/L of sodium sulfate accumulates during each turnover of the nickel concentration.
A nickel-phosphorus alloy is deposited on catalytic surfaces such as nickel or palladium. On other metal surfaces like steel, aluminum and copper alloys, a nickel immersion strike by galvanic contact or displacement must take place before catalytic nickel plating can begin. Plating will then continue, if pH and temperature are maintained until the nickel and/or hypophosphite are exhausted. Like all catalytic reactions, the process is easily poisoned or inhibited by certain metal impurities. The purity of the chemicals, especially the nickel sulfate, and the water quality are critical and should be monitored.
The lamellar (striated) deposits, which usually contain from 7 to 11 percent phosphorus, appear to be amorphous or noncrystalline, as determined by X-ray diffraction. The Ni-P alloys are considerably harder and more wear resistant than electroplated unalloyed nickel coatings. They are also less porous and have better resistance to salt spray. When heated in an oven to about 260°C, the coating is transformed into a crystalline mixture of nickel and nickel phosphite (Ni3P) with a gradual increase in hardness from 550 to 1000 Vickers. The properties of electroless nickel deposits have been described in detail.10
General considerations
A useful electroless nickel formulation must overcome a few principal problems:
1. Because about 100 g of sodium orthophosphite is generated for each mil-ft2 (25 μm-9.3 dm2) of Ni-P deposited, sparingly soluble nickel phosphite will precipitate unless the "free" nickel ion concentration is very low. This precipitate may cause bath decomposition and coating roughness. The proper kind and amount of nickel complexants can control the concentration of free nickel.
2. Hydrogen ions produced as a byproduct (Reaction 1) reduce bath pH and thereby the plating rate. In order to stabilize pH, the bath must contain buffers such as sodium salts of organic acids.
3. Inherent bath instability produces nickel fallout and plateout during plating. This must be minimized by the addition of stabilizers to prolong bath life and obtain maximum reducing efficiency from the hypophosphite.
Another important requirement is that the solution should have an initial plating rate of at least 13 μm/hr (0.5 mil/hr) at 90°C and pH 4.8. After several nickel turnovers, the rate should not be less than 10 μm/hr (0.4 mil/hr) and the bath should remain clear. The coating should be smooth and have a semibright appearance.
The amount of fallout and plateout in the plating tank should not require daily stripping with nitric acid. A 6-μm (0.25-mil) deposit on smooth steel should have 100-hr salt spray resistance. The phosphorus content of the deposit should be greater than 9% to obtain optimum coating properties such as low stress on steel.
In the last 10 years, the bath has been made more foolproof and easier to replenish. Most small plating tanks are equipped with electric immersion heaters; however, in large tanks, indirect or direct steam heaters are more efficient. Bag filters, as well as cartridges and precoat filters, are used for continuous filtration to remove all solid particles, which produce fallout and deposit roughness. Air agitation can reduce pitting in heavy deposits but increases hypophosphite consumption appreciably.
Bath components
A proper bath formulation is imperative for producing the highest-quality electroless nickel deposits. In this section, guidelines on nickel salts, hypophosphite, complexants, buffers, stabilizers, and other additives are given.
Nickel salt
Although nickel chloride** was used in the early baths, most recent solutions contain nickel sulfate in concentrations of 20 to 30 g/L (0.08 to 0.11 mol/L). A high nickel concentration gives a faster plating rate but bath stability is reduced. The nickel content determines the amount of hypophosphite and complexant required in the bath. To maintain a constant plating rate during operation, the nickel concentration should not decrease by more than 10 percent before replenishment.
Hypophosphite
The sodium salt has been used almost exclusively because it is readily available at a reasonable price. The optimum amount of hypophosphite depends primarily on the nickel concentration. Brenner used only 10 g/L in his unstabilized baths. Gutzeit, et al.11 patented a process with a nickel-to-hypophosphite molar ratio of about 0.25 to 0.6. The optimum ratio for achieving the best plating rate and deposit brightness was 0.3 to 0.4. In a bath containing 30 g/L of nickel sulfate (0.9 oz/gal Ni), the sodium hypophosphite concentration should be 30 to 40 g/L. At higher concentrations, the plating rate is faster, but stability is decreased. The hypophosphite must be replenished as often as the nickel to maintain the plating rate.
Based on operating experience, sodium hypophosphite consumption has been found to average 125 g per 100 g of nickel sulfate consumption. Thus, for each mil-ft2 of Ni-P, 100 g of hypophosphite is consumed. Only one-third of the hypophosphite is utilized in Ni-P deposition; the balance produces hydrogen gas (Reaction 3).
To maintain the proper ratio of nickel to hypophosphite, a daily hypophosphite analysis by iodine titration is strong recommended. With low tank loading and/or air agitation, hypophosphite consumption can be relatively high compared with nickel usage.
Complexants
The amount of chelating agents or ligands needed in a formulation depends not only on the nickel concentration but on their chemical structure, functionality and equivalent weight. Each nickel ion in solution is weakly bound to six water molecules. When these are replaced with hydroxy, carboxyl or amine groups, a stable nickel complex is formed. If the complexant contains more than one functional group, a closed-ring nickel complex or chelate results through oxygen and/or nitrogen coordination bonds. Most of the chelating agents used in electroless nickel plating solutions are hydroxy organic acids, which form one or more water-soluble nickel-ring complexes. Due to steric hindrance and strain, there are limits on the number and size of rings around each nickel atom.
In a solution containing 0.1 M (0.8 oz/gal) nickel, about 0.3 mol/L of a difunctional (bidentate) complexant such as glycolic or lactic acid is necessary to complex all of the nickel ions. With a trifunctional (tridentate) chelating agent such as malic acid, 0.2 mol/L is sufficient. When several complexants are used, their relative concentrations can best be optimized by experimentation. The objective is to minimize the free nickel ion concentration and still obtain a reasonably fast plating rate.
A wide choice of useful compounds at reasonable prices is not available. The most frequently used are glycolic (70%), lactic (80 and 88%), and malic and citric acids or their sodium salts. The relative amounts can vary considerably and affect not only the plating rate and bath stability but deposit properties.12 A few formulations contain gluconic acid or borogluconic acid.13
Glycine (aminoacetic acid) is a strong ligand employed mostly in neutral (pH 6 to 8) plating baths. Many proprietary solutions contain ammonium hydroxide, which forms a blue nickel complex in alkaline baths.
After several nickel turnovers, the complexant concentration in the bath must be increased to prevent it from turning cloudy due to nickel phosphite formation. Proprietary nickel replenishers normally contain sufficient complexants to make up for losses due to dragout and also to increase the complexant concentration in the bath. However, if the bath turns cloudy when heated, the addition of lactic or glycolic acid should be tried. Once the phosphite concentration exceeds 1.5 mol/L (five Ni turnovers), it is very difficult to maintain bath clarity and still plate at a reasonable rate.
Buffers
Because hydrogen ions are a byproduct of nickel reduction, the pH of the bath and therefore its plating rate decrease continuously unless the pH is stabilized with buffers (NaA), which combine with H+ as follows:
NaA + H+ ⇒ HA + Na+ (4)
The most effective compounds are the sodium or potassium salts of mono and dibasic organic acids. Only short-chain acids with two to six carbon atoms are suitable, because acids having higher molecular weights form insoluble nickel salts. The most frequently used monobasic acids are acetic and propionic or their salts. Dibasic acids such as adipic and succinic are also very effective buffers. Malonic acid is too expensive for commercial applications.
As discovered by Gutzeit,5,14 these acids also accelerate (exalt) the plating rate. Grobunova15 was the first to point out the need for a high ratio of buffer to hypophosphite with increasing hypophosphite concentration. Others have found that the succinate can exert either an accelerating or inhibitive effect depending on concentration.16 Some formulations contain both mono and dibasic acids. Because acetic and propionic acids are volatilized at plating temperatures, they require frequent replenishment. Most baths contain from 10 to 20 g/L of buffering compounds.
Despite the presence of buffers, the bath pH may decrease by 0.2 or more units after several hours of plating. To counteract this, the hypophosphite replenisher may contain carbonates of the alkali metals (e.g., potassium carbonate), which produce CO2 gas when the replenisher is added to the hot bath. Sodium acetate and propionate will also raise the pH.
If the pH of the plating solution falls below 4.5, dilute ammonia or 10% caustic solution must be added with vigorous agitation after the bath has cooled down to avoid formation of nickel hydroxide precipitate and spontaneous decomposition of the bath.
Some electroless nickel applications require a deposit with a high phosphorus content and hence reduced stress or magnetic susceptibility. The phosphorus content can be maximized by decreasing the pH to 4.0; however, this will decrease the plating rate to 5 μm/hr.17 Strong complexing agents produce deposits with higher phosphorus contents than weak complexing agents. Increasing the hypophosphite concentration to 40 g/L is also advisable.
Alkaline baths are primarily used for plating non-conductive (plastic and ceramic) surfaces after suitable etching and activation. These ammonia baths are operated at 30 to 60°C and have relatively slow plating rates.
Stabilizers
To operate satisfactorily without the danger of spontaneous decomposition during plating, the bath must be stabilized. Otherwise, nickel fallout will accumulate and initiate bulk or random reduction of nickel. Heavy-metal stabilizers such as lead inhibit the formation and growth of these particles. The stabilizers also decrease plateout on the sides and bottoms of tanks. Only a trace (<1 ppm) can be used because larger amounts will cause misplating or even poisoning of the bath.
Small amounts (1 to 5 mg/L) of thiourea and its derivatives such as mercaptobenzothiazole (MBT) are sometimes used with lead. This combination makes the lead concentration much less critical and imparts excellent stability and a fast plating rate. However, traces of sulfur co-deposited with the nickel drastically reduce the salt spray resistance of the deposit. As discussed later, the presence of sulfur can be readily detected by immersion of the deposit in nitric acid. Some inorganic sulfur compounds such as thiosulfate and metabisulfite are also effective stabilizers. Some baths are stabilized with molybdate18 or iodate19 compounds. Unsaturated organic acids20 also increase stability. None of these has any effect on fallout and plateout tendencies.
Traces of cadmium noticeably brighten the deposit. Usually, less than 1 mg/L is used because larger amounts will cause misplating on part edges, which preferentially absorb heavy metals. One of the most difficult operating problems is adequate stabilizer replenishment because depletion varies with the size and shape of the parts being plated. If the stabilizer is consumed at an especially high rate during plating, extra additions of the compounds may be required.
Other additives
Small quantities of fluoride can increase the plating rate slightly and promote plating on aluminum when no zincate pretreatment is used. In larger amounts, fluoride increases the hardness and stress of the deposit.
The continuous generation of hydrogen gas bubbles on work surfaces can produce streaking and pitting unless the parts are mechanically moved during plating. A small amount (<0.1%) of anionic wetting agent (sulfonate] can accelerate the release of hydrogen bubbles by decreasing the surface tension of the solution. Larger amounts can cause misplating, pitting and excessive foaming.
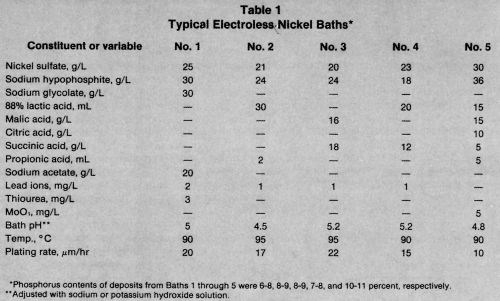
Five representative electroless nickel formulations are shown in Table 1. As discussed earlier, the pH and chemistry of the bath have an effect on the plating rate and phosphorus content of the deposit.
Experimental procedure
The effects of different organic acids and stabilizing compounds on plating rate, final pH, bath stability, and nitric acid resistance of deposits were investigated by making additions to a lactic acid bath.
Steel coupons (20 cm2) were plated for 1 hr using 200-mL solutions in large test tubes. Six of these were heated simultaneously in a controlled water bath. Plating rates were calculated from the weight gain of the coupons. After plating, the decrease in pH was measured with a pH meter at room temperature to determine the buffering capacity of the solution.
The palladium stability was determined by measuring the time to decomposition (i.e., the period required for the bath to turn black) after adding 2 mL of a 100-ppm palladium solution to 100 mL of the plating formulation at 60°C with agitation. For the nitric acid test the plated coupons were half immersed in concentrated acid for 60 sec.
The control plating solution was made up as follows: 35 mL/L of 88% lactic acid (USP), 13 g/L of sodium hydroxide, 30 g/L of nickel sulfate (purified), 25 g/L of sodium hypophosphite and 0 to 1 ppm of lead. The initial pH was 4.7.
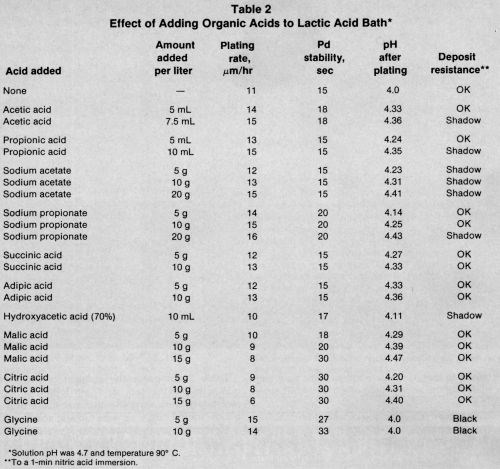
The effect of adding various organic acid complexants and buffers to the lactic acid bath is shown in Table 2 and the stabilizing effect of many compounds in Table 3. All plating was performed at 90±1°C or 93°C.
Results and discussion
The introduction of monobasic acids (neutralized with NaOH) or their sodium salts to the lactic acid bath accelerated plating in direct proportion to the amount added. They also buffered the solution during plating but had little effect on bath stability. The fastest deposition rate and highest post-plating pH was obtained with 20 g/L of sodium propionate. Sodium acetate decreased the nitric acid resistance of the deposit more than sodium propionate (Table 2).
The neutralized dibasic acids increased not only the plating rate by 20% but the buffering capacity of the lactic acid bath. They did not affect bath stability or nitric acid resistance. Because adipic and succinic acids have much lower volatility than acetic and propionic acids, the former are used in many formulations.
Hydroxy acids generally decreased the plating rate in direct proportion to their complexing strength and the amount added. Thus, hydroxyacetic (glycolic) acid had little effect while malic acid and especially citric acid decreased the rate considerably and increased the pH and bath stability. The nitric acid resistance of the deposit was diminished only by adding glycolic acid. Glycine (aminoacetic acid) increased the plating rate significantly, but the nitric acid resistance of the deposit was very poor.
Combinations of additives produced predictable results. The addition of 5 mL/L propionic and 10 g/L adipic acids increased the rate about 20%. The pH after plating was 4.5. When 10 g/L malic acid was also added, the plating rate decreased to that of the unmodified lactic acid bath, thereby nullifying the accelerating effect of the propionic and adipic acids.
The results show that a judicious combination of buffers and complexing agents gives the best balance of plating rate and pH stability. A strong complexant is required to prevent the precipitation of nickel phosphite, thereby prolonging the life of the bath.
The decrease in plating rate when lactic acid is partially replaced with a stronger complexant such as malic acid is illustrated in Fig. 1. The rate decreased from 15 to 12 μm/hr (0.58 to 0.48 mil/hr). With the addition of 10 mL/L propionic acid to these solutions, the rate increase averaged 10%. The effect was stronger on the solutions containing more lactic than malic acid. When 10 g/L adipic acid was added, the rate increase was 5 to 10%.
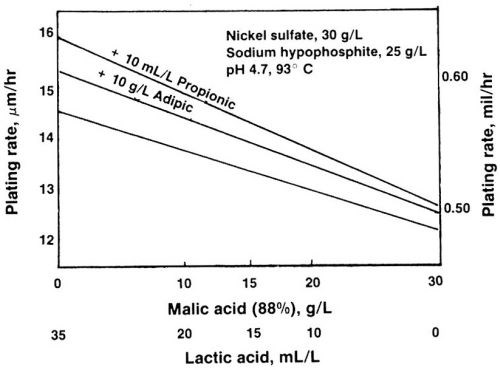
Figure 1 - Plating rate of electroless nickel baths containing malic and/or lactic acid.
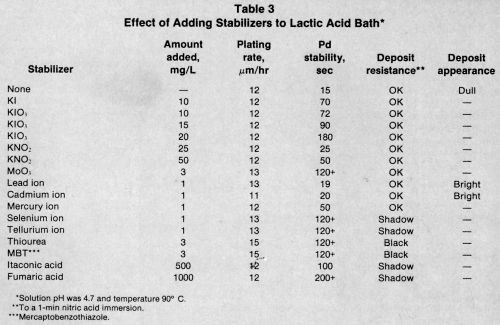
Table 3 compares the stabilizing effect of various compounds in the lactic acid bath. Iodine compounds and molybdate were very effective stabilizers over a certain concentration range, as were fumaric and itaconic acids.
Mercury, selenium, and tellurium produced better palladium stability than lead or cadmium. Only sulfur compounds increased the plating rate; however, they drastically reduced the nitric acid resistance of the deposit.
The amount of stabilizer the bath can tolerate varies with each compound and can only be determined by experimentation. The heavy-metal concentration is especially critical because more than 1 mg/L can cause misplating. Many proprietary baths contain more than one stabilizer to increase bath stability, prevent fallout, and impart deposit brightness.
Summary
Electroless nickel plating formulations are quite complex. Chelating agents, buffers and stabilizers affect the plating rate, bath stability and life, as well as the deposit properties. All plating chemicals must be carefully evaluated to determine their effects on the process and the coating. By selecting the appropriate components, an experienced formulator can tailor the bath to achieve the desired properties for a given application. However, a compromise such as slower plating rate may be necessary to ensure minimum porosity.
Because electroless nickel deposition is a chemical process, the bath requires more analytical control than most electroplating solutions. In order to obtain consistent plating rates and good electroless nickel deposits, the bath must be operated under optimum pH and temperature conditions with frequent replenishment as required by workload.
Some additives can promote certain coating properties such as brightness and smoothness. The plater now has a choice of proprietary baths to obtain, for instance, superior salt spray resistance at minimum coating thickness or low magnetic susceptibility for computer shielding applications.
References
1. A. Brenner and G. Riddell, Proc. AES Ann. Tech. Conf., 33, 16 (1946).
2. A. Brenner and G. Riddell, ibid., 34, 156 (1947).
3. G. Gutzeit, Trans. Inst. Met. Fin., 33, 383 (1956).
4. U.S. Patent 2,822,293 (1958).
5. U.S. Patent 2,822,294 (1958).
6. U.S. Patent 2,762,723 (1956).
7. G. Gutzeit, Plating, 46, 1158, 1275, 1377 (1959); 47, 63 (1960).
8. G. Salvago and P.L. Cavallotti, ibid., 59, 665 (1972).
9. V.A. Lloyd and G.O. Mallory, Proc. AES 1st Electroless Plating Symp., St. Louis (1982).
10. W.H. Safranek, The Properties of Electrodeposited Metals and Alloys, AESF, Orlando, FL, 1986; Ch. 23.
11. U.S. Patent 2,658,841 (1953).
12. G.O. Mallory, Plating, 61, 1005 (1974).
13. C.A. Boose, Trans. Inst. Met. Fin., 53, 49 (1975).
14. U.S. Patent 2,658,842 (1953).
15. K.M. Grobunova and A.A. Nikiforova, Physicochemical Principles of Nickel Plating, OTS-63-11003, U.S. Dept. of Commerce, Washington, DC, 1963; pp. 34-40.
16. K.A. Holbrook and P.J. Twist, Plating, 56, 523 (1969).
17. K. Parker and H. Shah, ibid., 58, 230 (1971).
18. U.S. Patent 2,876,116 (1959).
19. U.S. Patent 3,719,508 (1973).
20. U.S. Patent 3,782,978 (1974).
About the author (as written in 1987)

Konrad Parker, a specialist in electroless nickel plating, is a consultant headquartered at 1847 Stewart Ave., Park Ridge, IL. He formerly was group leader of Coating Research at the General American Research Div. of GATX Corp., Chicago, IL, where he was primarily responsible for the development of electroless nickel processes. He has also held various positions involving product development and application research of synthetic resins, photosensitive reproduction materials and chemical specialties for the plastic, rubber, lubricant and coating industries. A graduate of Illinois Institute of Technology with an M.S. degree, Mr. Parker is a member of the AESF Chicago Branch, Committee B-8 of ASTM, and the American Chemical Society. He has been granted many patents and has written numerous papers, including "Effects of Heat Treatment on the Properties of Electroless Nickel Deposits," for which he received the AESF Silver Medal Award in 1981.
* Developed by General American Transportation (GATX) Corp., Chicago, Illinois; now handled by Electro-Coatings, Moraga, California.
** The chloride increases tensile stress in the Ni-P deposit and is used in alkaline baths.
RELATED CONTENT
-
Masking for Surface Finishing
Masking is employed in most any metal finishing operation where only a specifically defined area of the surface of a part must be exposed to a process. Conversely, masking may be employed on a surface where treatment is either not required or must be avoided. This article covers the many aspects of masking for metal finishing, including applications, methods and the various types of masking employed.
-
An Overview of Electroless Nickel Plating
By definition, electroless plating is metal deposition by a controlled chemical reaction.
-
Gold and Silver Plating Basics
An overview of precious metal electroplating processes.


















