Titanium vs. Lead Anodes in Hard Chrome Plating
Platinized anodes optimize dimensioning and construction for hard chrome plating.
Lead is under a watchful eye across the globe. In the U.S., health and workplace authorities are insistent in their warnings. Even if electroplating companies have decades of experience working with hazardous substances, the metal is still being seen in an increasingly critical light.
For example, anyone using lead anodes in the U.S. must register with the federal EPA’s Toxic Chemical Release Inventory. If an electroplating company processes only around 29 kg of lead in a year, registration is still required.
Featured Content
So, it is not only hard chrome platers that need to seek alternatives in the U.S. Lead anodes may seem cheap at first glance but also have a wide spectrum of disadvantages:
- They can deform (see Figure 1). The isolated metal, therefore, cannot be equally distributed on the component. The layer thickness varies: Too much chrome is applied to some areas (waste of resources) and must be mechanically removed later.
- Maintenance costs are high. Repeated manual turning of the heavy lead anodes is common. This prevents, for example, short circuits via contact between anode and cathode.
- PbCrO4 also builds up on the anode. If the power supply is interrupted, lead chromate sludge can build up on the anode, and it can crumble when the power is restored. Lead chromate sludge poses a health risk. Disposing of this is time-consuming and expensive.
- Handling lead chromate sludge is very dubious from a work safety point of view. Therefore, it requires appropriately high levels of protective measures.
Dimensionally Stable Anodes
Dimensionally stable anodes are an interesting alternative for hard chrome plating (see Figure 2), with a platinum surface on titanium or niobium as the base substrate.
Platinum-plated anodes offer many benefits in hard chrome plating. This includes the following benefits, among others:
- Processes with dimensionally stable anodes are almost completely free of lead and therefore considerably more environmentally friendly. The result: no lead chromate, fewer health risks for workers and no disposal costs.
- Production downtime and exchanges are not required, as with lead systems.
- Pt/Ti and Pt/Nb anodes normally do not change their shape within the usual three-year period of use.
- The layer thicknesses on the cathodic components are even. Mechanical reworking is unnecessary.
- The amount of energy required is considerably lower than with lead anodes. In the long term, the voltage difference (e.g. change = around -1V with Pt/Ti anodes) means significantly lower costs. The reason for this is the anodic oxygen surge, which is much higher with lead than with platinum.
- Components such as power supply carriers and frame structures made from CuTi can be reused several times. This means that the time taken for the method to pay off is drastically lower when compared with lead anodes.
- Leftover platinum recovered at the end of the re-coating means that some original precious metal costs are recovered.
To achieve perfect results, adapt anodes to the construction of components to be coated. This makes dimensionally stable anodes possible (plates, cylinders, T-shapes and U-shapes), whereas lead anodes are mostly standardized sheets or rods.
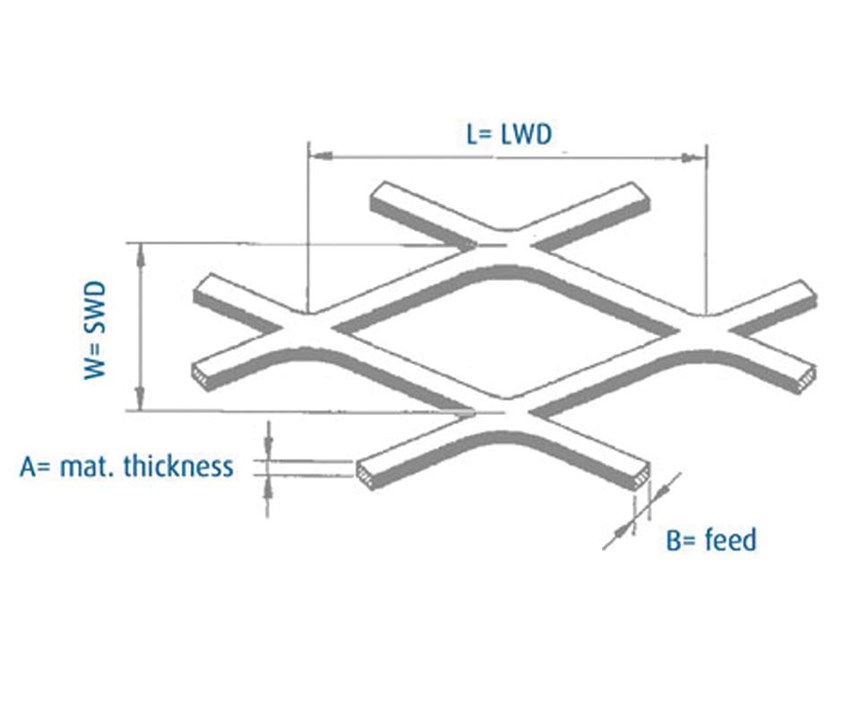
Pt/Ti and Pt/Nb anodes don’t have a closed surface, but are expanded metal sheets with variable mesh sizes. This leads to good power distribution; the electric field can work in and around the mesh (see Figure 3).
In coordination with the client, further optimizations can be carried out:
- To maximize the separation conditions in the electrolyte, an optimized mesh size is used (see Figure 4 and Table 1).
- This enables better gas removal as well as stronger electrolyte movement because of turbulent flow in the mesh.
A higher coating flux density is therefore possible with smaller distances between anodes and cathodes.
Layers can be applied more quickly: Production output increases.
A significant improvement in separation conditions can be achieved using grids with a large effective surface area.
Optimum Physical, Chemical Parameters
By combining platinum and titanium, dimensional stability is possible. Both metals offer optimal parameters for hard chrome plating. Platinum has a very low specific electrical resistance of just 0.107 Ω×mm2/m. Lead’s value is almost twice as high (0.208 Ω×mm2/m). Titanium’s corrosion resistance is outstanding, however, in the presence of halides, this is reduced. The breakdown voltage for titanium in electrolytes containing chloride, for example, is between 10 and 15 V, depending on the pH value. This is significantly higher with niobium (35 to 50 V) and tantalum (70 to 100 V).
Titanium does have disadvantages when it comes to corrosion resistance in strong acids, such as sulphuric, nitric, hydrofluoric, oxalic and methane sulphonic acids. However, titanium is still a good option because of its mechanical workability and price.
Application of the platinum layer on the titanium base substrate is best carried out electrochemically in molten salt via high-temperature electrolysis (HTE). The sophisticated HTE process offers precise coating: In a molten bath of 550°C, made with a mixture of potassium and sodium cyanide, with around 1- to 3-percent platinum, the precious metal is deposited electrochemically onto the titanium. The substrate is locked into a closed system with argon, where the salt bath is in a double crucible. Currents between 1 and 5 A/dm2 allow isolation rates between 10 and 50 μm per hour at a coating tension of 0.5 to 2 V.
The anodes that were platinum-plated using the HTE process are clearly superior to those coated in aqueous electrolytes. The purity of the molten salt platinum coating is at least 99.9 percent and therefore significantly higher than the purity of platinum layers deposited from aqueous solutions. Ductility, adhesion and corrosion resistance are significantly improved and inner tensions are minimal.
When considering the optimum anode construction, optimizing the anode’s carrier construction and power supply is of the utmost importance. The best solution is when a titanium sheeting coat is warmed and wrapped around a copper core. Copper is an ideal conductor and only has a specific electrical resistance of around 9 percent of a Pb/Sn alloy. The CuTi power supply only causes minimal power loss along the anode, so the layer thickness distribution on the cathodic component is equal.
Another positive effect is the formation of less heat. The need for cooling is reduced, as well as the platinum wear on the anode. The corrosion resistant titanium coating protects the copper core. When re-platinizing the expanded metal sheet, frame constructions and/or power supplies are only cleaned and prepared. These can be reused several times.
Avoiding Construction Errors
CuTi power supplies offer many possibilities for error:
- Too few welding spots can cause high transition resistance.
- Problems occur if the bracket construction in the power supply is not ideally constructed and is not divided appropriately.
- With fluoride containing Cr6 electrolytes, it is extremely important to choose a suitable base material for the anode.
- There should be no TIG/laser welding zones in the plating area: Melting the Pt/Ti coating system onto the welding join must be avoided. This could otherwise lead to alloy build-up, which reduces the corrosion resistance. In addition, heat weakens the adhesion of the Pt layer (peel-off effect).
- Finally, heat develops around a defective welding spot. The result is premature wear.
As long as these construction recommendations are followed, you will create the “perfect anode” for hard chrome plating, with Pt/Ti or Pt/Nb models. Dimensionally stable models are more expensive in the investment phase than lead anodes. But when costs are considered in more detail, titanium models with a platinum surface could be an interesting alternative for hard chrome plating.
This is explained by a comprehensive and thorough analysis of total costs for conventional lead anodes and platinum anodes.
Eight lead alloy anodes made from PbSn7, with a length of 1,700 mm and a diameter of 40 mm, were compared with the appropriately dimensioned Pt/Ti anodes for chrome plating a cylindrical component.
Manufacturing costs for the eight lead anodes came to around 1,400 euros ($1,471), which seems cheap at first glance. The investment required to develop the necessary Pt/Ti anodes is considerably higher. This came to around 7,000 euros for the initial purchase. The platinum coating is especially costly. The pure precious metals alone make up 45 percent of this amount. The platinum coating of 2.5 μm requires 11.3 g of the precious metal for each of the eight anodes. At a price of 35 euros per gram, this makes 3,160 euros.
Minimize Production Downtime
Even though lead anodes seem the better choice, this changes quickly on closer inspection.
After just three years, the total cost for the lead anodes is considerably higher than for the Pt/Ti models. In the conservative example calculation, a typical application flow density of 40 A/dm2 is assumed. In the result, there is a power flow of 6,720 amperes for the given anode surface of 168 dm2 during a running time of 6,700 hours in three years. This corresponds to 10 hours of net operational time on roughly 220 working days per year. The platinum layer thickness decreases slowly because of platinum oxidation going into solution. In the example, this was taken into account as 2 grams per 1 million ampere hours.
There are many reasons behind the cost benefits of Pt/Ti in comparison with lead anodes. Most importantly, the reduced electricity usage (minus 14,800 kWh/year at a price of 0.14 euro/kWh) costs around 2,000 euro per year. Additionally, the roughly 500 euros in yearly disposal costs for lead chromate sludge and 1,000 euros for maintenance and production downtime are no longer required— and this was very conservatively calculated.
Total costs for lead anodes over three years came to 14,400 euros ($15,130). For Pt/Ti anodes, costs were 12,020 euros—including re-platinization. Even if the costs for maintenance and production downtime (1,000 euros for one day per year) weren’t accounted for, the break-even point would still be reached after three years. From this time on, the gap between the two widens further, in favor of the Pt/Ti anodes.
Many branches take advantage of the various benefits of high-temperature electrolyzed platinized anodes. Manufacturers in the lighting, semi-conductor and PCB, automotive, hydraulics, mining machinery, waterworks and swimming pool industries all rely on these coating technologies. More applications will surely be developed in the future, as sustainable cost considerations and environmental protection are long-term concerns. Therefore, lead may face even more critical scrutiny.
The original text was published in German in Annual Surface Technology (Volume 71, 2015), edited by Professor Timo Sörgel, University of Applied Science, Aalen/Germany. Courtesy of Eugen G. Leuze Verlag, Bad Saulgau/Germany.
RELATED CONTENT
-
Gold and Silver Plating Basics
An overview of precious metal electroplating processes.
-
Zinc Electroplating
Choosing the best process for your operation.
-
Aluminum Anodizing
Types of anodizing, processes, equipment selection and tank construction.