NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 8th Quarterly Report
This eighth quarter report covers work to prepare and characterize surface-fluorinated Ti4O7 anodes to enhance further the PFAS degradation process.
Share
8th Quarterly Report
October-December 2022
AESF Research Project #R-122
Electrochemical Approaches to Treatment of PFAS in Plating Wastewater
by
Qingguo (Jack) Huang* and Yuqing Ji
College of Agricultural and Environmental Science
University of Georgia
Griffin, GA, USA
Editor’s Note: For 2021, NASF-AESF Foundation Research Board selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the eighth quarter of work (October-December 2022). A printable PDF version of this report is available by clicking HERE.
1. Introduction
This project started in January 2021 with the goal of developing applicable electrochemical approaches to remove per- and polyfluoroalkyl substances (PFASs) present in plating wastewaters, including electrooxidation (EO) and electrocoagulation (EC). This project includes three research tasks that are designed to investigate EC, EO and EC-EO treatment train, respectively, designed to probe three hypotheses specified follows:
1) EC generates amorphous metal hydroxide flocs that can effectively adsorb PFASs in plating wastewater, which, through an appropriate treatment, can release PFASs into a concentrated solution.
2) EO enabled by a Magnéli phase Ti4O7 anode can be used to effectively destruct PFASs in plating wastewater.
3) The electrochemical treatment train comprised of EC and EO by Ti4O7 anode can remove and degrade PFASs in plating wastewater more efficiently than either process operated individually.
In this quarterly report, we describe a work to prepare and characterize surface-fluorinated Ti4O7 anodes. This was attempted because it was reported that surface fluorination of an anode can usually increase its hydrophobicity and thus enhance its efficiency in degrading organic compounds during electrooxidation (EO), as well as reduce the formation of perchlorate (ClO4-) formation. The performance of the surface-fluorinated Ti4O7 anodes in degrading model PFAS will be reported in ensuing reports.
2. Experimental
2.1 Chemical and reagent preparation
All chemicals used in the experiments were reagent grade and used as received. Polytetrafluoroethylene (PTFE) preparation (60 wt% dispersion in H2O was obtained from Sigma Aldrich (St. Louis, USA). Sodium sulfate (Na2SO4) and ammonium acetate (CH3COONH4) were obtained from J.T. Baker Chemical (Phillipsburg, USA). Sodium perchlorate (NaClO4), sodium chloride (NaCl) and HPLC-grade methanol were purchased from Fisher Scientific (Pittsburgh, USA). Sodium fluoride (NaF, 99%) was obtained from Alfa Aesar (Tewksbury, USA).
2.2 Anodes Fabrication and Fluorination
In this experiment, Magnéli phase Ti4O7 anodes were used, which were fabricated through high-temperature sintering and cut from one plate into smaller circular plates of the same size (2.2-cm diameter). The anodes were then surface fluorinated using an electrodeposition procedure in a reactive electrochemical membrane (REM) reactor.
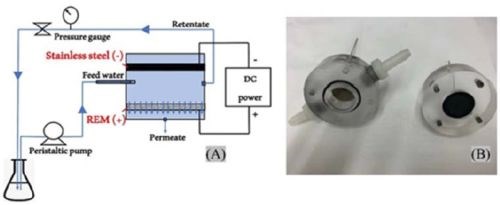
Figure 1 - (A) A schematic diagram of REM reactor with Ti4O7 anode in cross-flow filtration operation; (B) a picture of actual reactor.
The reactor is shown in Figure 1. It consists of a cylindrical vessel with a Ti4O7 anode and a circular stainless-steel cathode installed in parallel with a gap of 1.20 cm. There are inlet and outlet ports for the sample solution to flow through the space between the electrodes, and another outlet port is present on the anode side to collect the permeate. The retentate flow was recycled, while the permeate flow was not. The permeate flux was regulated by a back pressure regulator, and the flow rate was kept constant at 0.2 cm/min. Surface fluorination was performed in cross-flow mode, with a pristine Ti4O7 plate as the anode. The feed solution contained 100-mM NaClO4 as the supporting electrolyte, and polytetrafluoroethylene (PTFE) resin at 0.5, 1.0, 2.0 or 5.0 mL/L, respectively with 10, 20, 30 or 40 mM of NaF. The pH of all deposition solutions was adjusted to 3.5. The REM operation was conducted for 60 min with a current density of 40 mA/cm2 applied and the feed solution heated to 85°C in a water bath to facilitate electrodeposition of fluorine on the anode surface. The surface-fluorinated Ti4O7 anodes were then stabilized at 150°C in a muffle furnace (KSL-1200X) (MTI Corporation, CA) overnight. Tests were conducted to confirm that no fluoride ion was released during electrooxidation in 100-mM Na2SO4 solution at 20 mA/cm2.
3. Results and discussion
X-Ray photoelectron spectroscopy (XPS) was performed to examine the results of surface fluorination on the anodes performed by electrodeposition with solutions of varying NaF and PTFE concentrations (Table 1), and the spectra are displayed in Figure 2. Figure 2A presents the XPS full scan of pristine and fluorinated Ti4O7 anodes. The oxygen O 1s, titanium Ti 2p and carbon C 1s peaks were observed at 532 eV, 461 eV and 287 eV, respectively, and their atomic percentages are listed in Table 2. The fluorine peaks were found around 687 eV on fluorinated Ti4O7 anode surfaces, while it was absent on the pristine Ti4O7 anode. A detailed scan of fluorine atom (Fig. 2B) exhibits a major peak at 689.5 eV, corresponding to the C-F bond in CF3(-CF2-)n, and a minor peak at 684.9 eV, corresponding to the Ti-F bond (Beamson and Briggs, 1992). This indicates that both the fluoroalkyl segments of PTFE and F- were electrodeposited on the Ti4O7 surface, consistent with previous studies (Pei, et al., 2021).
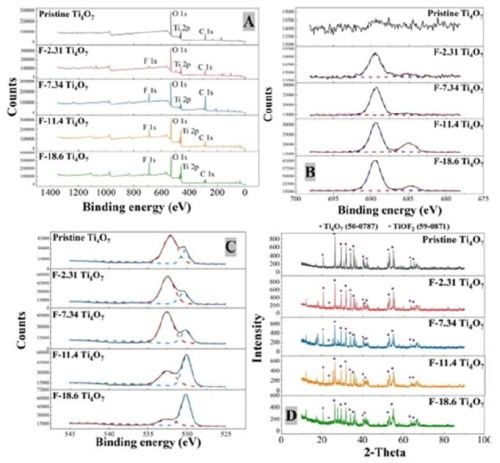
Figure 2 - The XPS analysis of pristine and surface fluorinated Ti4O7 anodes: (A) XPS survey, (B) detailed scan of F 1s, (C) O 1s and (D) XRD results. The purple dots and grey dots represent the characteristic peaks of ICDD Ti4O7 (50-0787) and TiOF2 (59-0871), respectively.
Based on the XPS data in Figure 2, the atomic percentages of F on Ti4O7 anodes, respectively in Ti-F and C-F form and the total F, are summarized in Table 1. As seen in Table 1, the atomic percentages of F on the Ti4O7 anodes were 2.31%, 7.34%, 11.4% and 18.6%, increasing along with the rising NaF and PTFE concentrations used in the electrodeposition solutions. The surface-fluorinated Ti4O7 anodes are then named according to their respective F percentage, i.e., F-2.31, F-7.34, F-11.4, or F-18.6 Ti4O7 (Table 1), respectively, from this point forward. Elemental mapping of F and C by EDS survey of the fluorinated Ti4O7 anode surface confirms the existence of CF3(-CF2-)n groups (Figure 3).
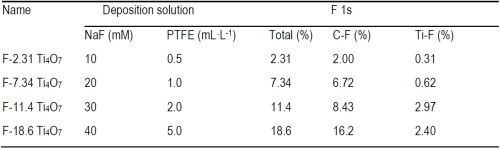
Table 1 - The atomic percentage (%) of different elements on pristine and fluorinated Ti4O7 anodes.
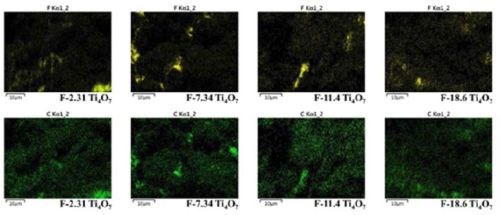
Figure 3 - The element mapping of fluorine (yellow) and carbon (green) on fluorinated Ti4O7 anodes.
The data in Table 1 also suggests that the C-F form dominates in the surface-fluorinated surface instead of Ti-F. Moreover, it is noted that the increasing F atom percentage (from 2.31% to 18.6%) corresponds to a decreasing O atom percentage (from 53.3% to 36.6%) on the anode surfaces (Table 2), indicated by the O 1s peak of 532.5 eV binding energy (Fig. 2C). This suggests that surface fluorination of Ti4O7 occurred by the substitution of -OH in the surface titanol group (≡TiOH) by F and CF3(-CF2-)n.
XRD was performed to characterize the pristine and fluorinated Ti4O7 anodes with standard reference materials to identify their compositions and phases (Fig. 2D). The XRD pattern of pristine and fluorinated Ti4O7 anodes are similar, primarily composed of Ti4O7. The diffractive peak at 2θ = 21.5° and 41.3° in fluorinated Ti4O7 anodes indicates the appearance of TiOF2 in Ti4O7 lattice.
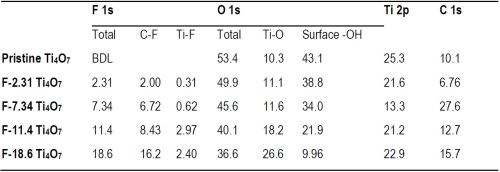
Table 2 - Composition of electrodeposition solution and corresponding atomic concentration of F on the Ti4O7 anode.
4. References
1. G. Beamson and D.R. Briggs, High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, J. Wiley & Sons, Chichester, UK, 1992.
2. S. Pei, H. Shi, J. Zhang, S. Wang, N. Ren and S. You, “Electrochemical removal of tetrabromobisphenol A by fluorine-doped titanium suboxide electrochemically reactive membrane,” Journal of Hazardous Materials, 419, 126434 (2021).
5. Past project reports
1. Introduction to Project R-122: Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (6), 13 (March 2021); Full paper: http://short.pfonline.com/NASF21Mar1.
2. Quarter 1 (January-March 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (12), 13 (September 2021); Full paper: http://short.pfonline.com/NASF21Sep1.
3. Quarter 2 (April-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (3), 18 (December 2021); Full paper: http://short.pfonline.com/NASF21Dec2.
4. Quarter 3 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (6), 16 (March 2022); Full paper: http://short.pfonline.com/NASF22Mar2.
5. Quarter 4 (October-December 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (9), 21 (June 2022); Full paper: http://short.pfonline.com/NASF22Jun2.
6. Quarter 5 (January-March 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (12), 22 (September 2022); Full paper: http://short.pfonline.com/NASF22Sep2.
7. Quarter 6 (April-June 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 87 (3), 17 (December 2022); Full paper: http://short.pfonline.com/NASF22Dec1.
8. Quarter 7 (July-September 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 87 (6), 19 (March 2023); Full paper: http://short.pfonline.com/NASF23Mar2.
6. About the principal investigator

Qingguo (Jack) Huang is Professor in the Department of Crop and Soil Sciences, University of Georgia, Griffin Campus. He holds a B.S. in Environmental Science (1990) and a Ph.D. in Chemistry (1995) from Nanjing University, China as well as a Ph.D. in Environmental Engineering from the University of Michigan, Ann Arbor, Michigan. Dr. Huang’s research interest focuses on catalysis involved in the environmental transformation of organic pollutants, and development of catalysis-based technology for pollution control and environmental remediation and management. His laboratory has been actively involved in several cutting-edge research topics:
- Enzyme-based technology for water/wastewater treatment and soil remediation
- Electrochemical and reactive electrochemical membrane processes in wastewater treatment
- Catalysis in biofuel production and agro-ecosystem management
- Environmental fate and destructive treatment methods of PFASs
- Environmental application and implication of nanomaterials
* Principal Investigator (PI) Contact Information: Qingguo Huang, Ph.D, Professor, Department of Crop and Soil Sciences, University of Georgia, 1109 Experiment St., Griffin, GA 30215, USA.
Phone: (770) 229-3302
Fax: (770) 412-4734
E-mail: qhuang@uga.edu
Related Content
NASF/AESF Foundation Research Project #123: Electrochemical Manufacturing for Energy Applications – 4th and 5th Quarter Report
The NASF-AESF Foundation Research Board selected a project on electrodeposition toward developing low-cost and scalable manufacturing processes for hydrogen fuel cells and electrolysis cells for clean transportation and distributed power applications. During the reporting period, efforts were focused on planning the overall project work, with the eventual goal of manufacturing an improved design for a Solid oxide fuel cell anode supported flat tube (SOFC).
Read MoreDevelopment of a Novel Hexavalent-Chromium-Free Aluminum Sacrificial Paint
Hexavalent chromium is a known carcinogen, repro-toxin, and mutagen. Its elimination is of high importance to the aerospace industry, which has struggled to find high performing alternatives. Legacy aluminum sacrificial paints have traditionally utilized hexavalent chromium to prevent corrosion and coatings which are equal to or better than have been difficult. This first paper discusses the novel process from the supplier point-of-view.
Read MoreNASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 12th Quarterly Report
This NASF-AESF Foundation research project report covers the 12th quarter of project work (October – December 2023) at the University of Georgia. In our previous report, we described our work on performance and effect of surface fluorinated Ti4O7 anodes on PFAS degradation in reactive electrochemical membrane (REM) mode. This quarter, our experiments involved utilizing porous Ti4O7 plates serving both as anodes and membranes. Tests compared pristine and F-18.6 Ti4O7 anodes at current densities of 10 mA/cm2 and 40 mA/cm2. This 12th quarterly report discusses the mechanisms of the effects on EO performance by anode surface fluorination.
Read MoreTin-Zinc Alloy Electroplating and Its Corrosion Behavior
An NASF/AESF Foundation Research Program Retrospective
Read MoreRead Next
Masking Solutions for Medical Applications
According to Custom Fabricating and Supplies, a cleanroom is ideal for converting, die cutting, laminating, slitting, packaging and assembly of medical-grade products.
Read MoreEducation Bringing Cleaning to Machining
Debuting new speakers and cleaning technology content during this half-day workshop co-located with IMTS 2024.
Read MoreDelivering Increased Benefits to Greenhouse Films
Baystar's Borstar technology is helping customers deliver better, more reliable production methods to greenhouse agriculture.
Read More





















