NASF/AESF Foundation Research Project #120: Electrochemical Destruction of Perfluorooctanesulfonate in Electroplating Wastewaters - April 2022-March 2023
This NASF-AESF Foundation research project report covers project work from April 2022 to March 2023 at the University of Illinois at Chicago. The overall objective of this work is to utilize a cost-effective reactive electrochemical membrane (REM) for the removal of PFAS from synthetic electroplating wastewater. Initial results for the oxidation of PFOA with three different catalysts are discussed.
#pollutioncontrol #nasf
by
Brian P. Chaplin*
Department of Chemical Engineering
University of Illinois at Chicago
Chicago, Illinois, USA
Editor’s Note: This NASF-AESF Foundation research project report covers the year of project work from April 2022 to March 2023) at the University of Illinois at Chicago. A printable PDF version of this report is available by clicking HERE. A listing of previous reports to date is provided at the end of this report.
Featured Content
Overview
1. Development of REMs for destructive PFAS removal in synthetic electroplating wastewater.
2. Determination of the optimal operational mode.
3. Calculation of energy requirements for the REM-based system and compare to those determined for GAC adsorption and other technologies.
Achieving these objectives will provide the necessary data to determine if the REM system is competitive with other treatment options and thus will allow for the pursuit of further funding from industry and other funding agencies. Specific technical questions are stated below.
Question 1: Can adsorbent materials be added to REMs to produce next generation REMs with enhanced sorption capacities for PFAS?
Question 2: What is the best mode of operation for optimal REM performance for PFAS removal?
Question 3: Will the REMs be a technically effective and cost-efficient remediation strategy for PFAS-containing electroplating wastewater?
Summary: 9th thru 11th Quarters (April-December 2022)
During this reporting period, a no-cost extension year was required owing to the temporary closure of the laboratory related to COVID issues. Due to this situation and the fact that there was not a student available to work on the project, work was placed on hold. The search for a new student was still underway, and the research would continue at that time.
Summary: 12th Quarter (January-March 2023)
For the beginning of 2023, work resumed. A new student was hired on this project and has spent time learning the experimental setup and appropriate methods. In addition, a new catalytic reactor, which was developed on another project, will be tested in the next quarter for the degradation or PFAS in controlled samples and electroplating wastewater. Initial results for the oxidation of PFOA with this catalyst are shown below.
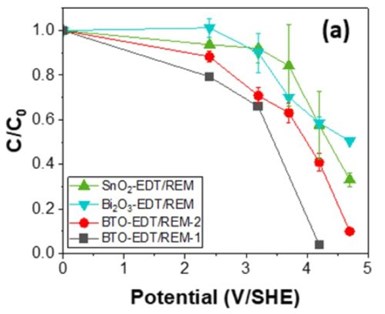
Figure 1: C/C0 profile of PFOA (C0 = 100 μM) as a function of potential.
Figure 1 shows the concentration profile of PFOA at different potentials using three different catalysts: (1) a SnO2 catalyst deposited by electrodeposition, followed by thermal oxidation (EDT) (i.e., SnO2-EDT/REM); (2) a Bi2O3 catalyst deposited by EDT (i.e., Bi2O3-EDT/REM); and (3) two Bi2O3-SnO2 catalyst (BTO) deposited by EDT (i.e., BTO-EDT/REM). At 4.2 VSHE, 42.4 ± 15.3%, 41.4 ± 2.6%, 59.0 ± 4.1% and >90% removal of PFOA was observed using SnO2-EDT/REM, Bi2O3-EDT/REM, BTO-EDT/REM-2, and BTO-EDT/REM-1, respectively. Overall, the results showed higher removal of PFOA for SnO2-EDT/REM compared to Bi2O3-EDT/REM. However, we observed that SnO2 was leaching into the permeate solution at potentials ≥ 3.7 VSHE. From the concentration profiles for BTO-EDT/REMs, it can be observed that presence of Bi2O3 improves removal of PFOA. We hypothesize that Bi2O3 stabilizes SnO2.
Past project reports
1. Quarters 1-5 (April 2019-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (1), 19 (October 2021); Full paper (With Project Introduction): http://short.pfonline.com/NASF21Oct1.
2. Quarter 6 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (4), 19 (January 2022); Full paper: http://short.pfonline.com/NASF22Jan2.
3. Quarters 7-8 (October 2021-March 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (11), 19 (August 2022); Full paper: http://short.pfonline.com/NASF21Aug2.
About the principal investigator

Dr. Brian P. Chaplin is a Professor in the Department of Chemical Engineering, at the University of Illinois at Chicago. He holds a B. Civil Engineering (1999) and an M.S. (2003) in Civil Engineering from the University of Minnesota and a Ph.D. in Environmental Engineering (2007) from the University of Illinois at Urbana-Champaign.
*Corresponding Author (Principal Investigator)
Dr. Brian P. Chaplin, Professor
Dept. of Chemical Engineering
University of Illinois at Chicago
221 Chemical Engineering Building
810 S. Clinton St.
Chicago, IL 60607
Office: (312) 996-0288
Mobile: (217) 369-5529
E-mail: chaplin@uic.edu
RELATED CONTENT
-
Plating Q&A: Can you color stainless steel?
Our expert, Art Kushner, says yes, you can color stainless steel, but it is not a process that is typically performed in a plating shop. Read more about his answer.
-
Treating Plating Wastewater
Wastewater from plating facilities contains contaminants such as heavy metals, oil and grease and suspended solids at levels that might be considered environmentally hazardous . . .
-
Zinc Phosphate: Questions and Answers
Our experts share specific questions about zinc phosphate and pretreatment


















