NASF/AESF Foundation Research Project #122: Electrochemical Approaches to Treatment of PFAS in Plating Wastewater - 7th Quarterly Report
The NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams, studying PFAS destruction via electrooxidation and electrocoagulation. Our last report described the results from experiments of EO with a Magnéli phase Ti4O7 anode on the degradation of eight perfluoroalkyl acids (PFAAs). In this seven quarter report, we describe work to further explore how the degradation of different PFAAs are related to their molecular structures.
#pollution control #nasf
by
Qingguo (Jack) Huang* and Yuqing Ji
College of Agricultural and Environmental Science
University of Georgia
Griffin, GA, USA
Editor’s Note: For 2021, NASF-AESF Foundation Research Board has selected a project on addressing the problem of PFAS and related chemicals in plating wastewater streams. This report covers the seventh quarter of work (July-September 2022). A printable PDF version of this report is available by clicking HERE.
Featured Content
Introduction
This project started in January 2021 with the goal of developing applicable electrochemical approaches to remove per- and polyfluoroalkyl substances (PFASs) present in plating wastewaters, including electrooxidation (EO) and electrocoagulation (EC). This project includes three research tasks that are designed to investigate EC, EO and EC-EO treatment train, respectively, designed to probe three hypotheses specified follows:
1) EC generates amorphous metal hydroxide flocs that can effectively adsorb PFASs in plating wastewater, which, through an appropriate treatment, can release PFASs into a concentrated solution.
2) EO enabled by a Magnéli phase Ti4O7 anode can be used to effectively destruct PFASs in plating wastewater.
3) The electrochemical treatment train comprised of EC and EO by Ti4O7 anode can remove and degrade PFASs in plating wastewater more efficiently than either process operated individually.
Our previous report describes the results from experiments of EO with a Magnéli phase Ti4O7 anode on the degradation of eight perfluoroalkyl acids (PFAAs), including perfluorobutanoic acid (PFBA), perfluopentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS) and perfluorooctanesulfonic acid (PFOS). Here in this report, we describe a work to further explore how the degradation of different PFAAs are related to their molecular structures.
Experimental
In this work, a suite of molecular descriptors was calculated based on quantum mechanical computation. Then, the relationships between the rate constants of PFAA degradation and the molecular descriptors were explored in this study. Measurement of the PFAA degradation rate constants was described in our last report, and the EO was conducted in solutions each containing an individual PFAA at the current density of 5 mA/cm2. Quantum computation was performed using Gaussian 09 based on the density functional theory (DFT) at B3LYP/6-311G** level. The molecular geometry was optimized, and vibrational frequencies were computed for each PFAA, based on which a suite of molecular descriptors were calculated, including the frontier electron densities (FEDs) of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), the energy level of HOMO (EHOMO) and LUMO (ELUMO) and their gap (ELUMO-EHOMO), and molecular polarizabilities.
Results and discussion
The degradation rates of the eight PFAAs by EO were found to be very different in the study described in our last report. The relationships between the rate constants of PFAA degradation normalized to the effective electroactive surface area (EEAA) and the molecular descriptors were explored. It has been proposed that the combination of direct electron transfer and hydroxyl radical attack plays a major role in the electro-oxidative degradation of PFAA.1 Therefore, certain molecular and electrical distribution properties of the PFAAs relating to their reactivity with hydroxyl radicals and electron transfer have been examined.
Frontier molecular orbital energies often play an important role in determining reactivity of organic chemicals.2 Energy of the highest occupied molecular orbital (Ehomo) and the lowest unoccupied molecular orbital (Elumo) represents the ability of a molecule to donate or gain an electron respectively, and the gap between them imply the stability of the molecule in terms of redox reactivity.3,4 In this study, greater ELUMO-EHOMO gaps were seen for shorter chained PFAAs ranging from 0.244 hartrees for PFBA to 0.155 hartrees for PFOA. It thus makes sense that we have seen a negative correlation between the ELUMO-EHOMO gap and the log kSA (Fig. 1A). Indeed, these values form a cohesive negative linear trend through each of the functional groups (PFSAs vs. PFCAs) when plotted log kSA against the ELUMO-EHOMO gap. The energy gap however, does not capture the rate constant trend between the functional groups, although this is not surprising, given the large difference that functional groups can make on molecular properties.
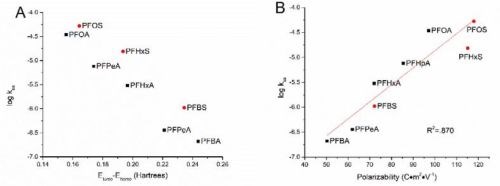
Figure 1 - Relationship between the logarithm of the surface area normalized rate constants and (A) ELUMO-EHOMO gap and (B) molecular polarizability.
Another molecular descriptor of interest, molecular polarizability, indicates the electronic flexibility of each molecule, thus in connection to the tendency of oxidative reactivity as well as direct electron transfer on the anode.5 When plotted against the PFAA degradation rate constants (Fig. 1B), the molecular polarizability has a nice positive correlation across both functional groups, serving as a good indicator for the reactivity of PFAAs. This suggests that direct electron transfer may be the rate-limiting step in PFAA degradation in TSO-based electro-oxidation systems, although the hydroxyl radicals generated via water oxidation may also play a role in concert.1
References
1. H. Lin, J.F. Niu, S.T. Liang, C. Wang, Y.J. Wang, F.Y. Jin, Q. Luo and Q.G. Huang, “Development of microporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances,” Chem. Eng. J., 354, 1058-1067 (2018).
2. Z.W. Cheng, B.W. Yang, Q.C. Chen, W.C. Ji and Z.M. Shen, “Characteristics and difference of oxidation and coagulation mechanisms for the removal of organic compounds by quantum parameter analysis,” Chem. Eng. J., 332, 351-360 (2018).
3. H. Kusic, B. Rasulev, D. Leszczynska, J. Leszczynski and N. Koprivanac, “Prediction of rate constants for radical degradation of aromatic pollutants in water matrix: A QSAR study,” Chemosphere, 75 (8), 1128-1134 (2009).
4. C.G. Zhan, J.A. Nichols and D.A. Dixon, “Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: Molecular properties from density functional theory orbital energies,” J. Phys. Chem. A, 107 (20), 4184-4195 (2003).
5. X.H. Jin, S. Peldszus and P.M. Huck, “Predicting the reaction rate constants of micropollutants with hydroxyl radicals in water using QSPR modeling,” Chemosphere, 138, 1-9 (2015).
Past project reports
1. Introduction to Project R-122: Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (6), 13 (March 2021); Full paper: http://short.pfonline.com/NASF21Mar1.
2. Quarter 1 (January-March 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 85 (12), 13 (September 2021); Full paper: http://short.pfonline.com/NASF21Sep1.
3. Quarter 2 (April-June 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (3), 18 (December 2021); Full paper: http://short.pfonline.com/NASF21Dec2.
4. Quarter 3 (July-September 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (6), 16 (March 2022); Full paper: http://short.pfonline.com/NASF22Mar2.
5. Quarter 4 (October-December 2021): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (9), 21 (June 2022); Full paper: http://short.pfonline.com/NASF22Jun2.
6. Quarter 5 (January-March 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 86 (12), 22 (September 2022); Full paper: http://short.pfonline.com/NASF22Sep2.
7. Quarter 6 (April-June 2022): Summary: NASF Report in Products Finishing; NASF Surface Technology White Papers, 87 (3), 17 (December 2022); Full paper: http://short.pfonline.com/NASF22Dec1.
About the author

Qingguo (Jack) Huang
Qingguo (Jack) Huang is Professor in the Department of Crop and Soil Sciences, University of Georgia, Griffin Campus. He holds a B.S. in Environmental Science (1990) and a Ph.D. in Chemistry (1995) from Nanjing University, China as well as a Ph.D. in Environmental Engineering from the University of Michigan, Ann Arbor, Michigan. Dr. Huang’s research interest focuses on catalysis involved in the environmental transformation of organic pollutants, and development of catalysis-based technology for pollution control and environmental remediation and management. His laboratory has been actively involved in several cutting-edge research topics:
- Enzyme-based technology for water/wastewater treatment and soil remediation
- Electrochemical and reactive electrochemical membrane processes in wastewater treatment
- Catalysis in biofuel production and agro-ecosystem management
- Environmental fate and destructive treatment methods of PFASs
- Environmental application and implication of nanomaterials
He has published over 170 peer-reviewed journal articles, five book chapters and four patents and three patents pending. He has taught three courses at the University Georgia: Introduction to Water Quality, Environmental Measurement and Advanced Instrumental Analysis in Environmental Studies.
* Principal Investigator (PI) Contact Information:
Qingguo Huang, Ph.D, Professor, Department of Crop and Soil Sciences, University of Georgia
1109 Experiment St., Griffin, GA 30215, USA.
Phone: (770) 229-3302
Fax: (770) 412-4734
E-mail: qhuang@uga.edu
RELATED CONTENT
-
NOx Scrubbing Technology Breakthrough
This paper presents research findings and practical results that address the treatment of the problematic greenhouse gases nitrogen oxides (NOx) and sulfur dioxide (SO2).
-
Cleaning Magnesium
Question: What is the recommended chemical cleaning process and composition prior to electroless nickel plating for magnesium?
-
Nitric Acid Passivation and EH&S Impact
I am looking for safety/environmental requirements to set up a nitric acid cleaning and passivation system.


















