Phosphate Conversion Coatings
Types of phosphate conversion coatings, how to apply them, and their specific functions.
#basics
Convert: To change into another form, substance or state. In the case of phosphate conversion coatings, the substrate metal participates in the coating reaction and becomes a component of the coating. Phosphate conversion coatings are formed from the surface of the base metal outward. Therefore, the thickness of the coating is dependent upon the porosity of the coating as it forms. Once the surface is sealed from the chemical solution, the reaction stops.
Phosphate conversion coatings are an integral part of most finishing operations, serving one or more of the following functions: increase corrosion resistance, absorb lubricants, enhance appearance, promote adhesion, and provide wear resistance or facilitate cold forming.
Featured Content
Phosphating is a chemical conversion coating that transforms the surface of the basis metal into a non-metallic crystalline coating. The reaction occurs in an acidic solution containing phosphate ions. Loss of hydrogen at the metal/solution interface results in a localized rise in pH and subsequent precipitation of the coating.
Phosphate coatings can be categorized into three main types: zinc, manganese and iron. There are many proprietary formulations available for each, depending on the functional requirements of the part.
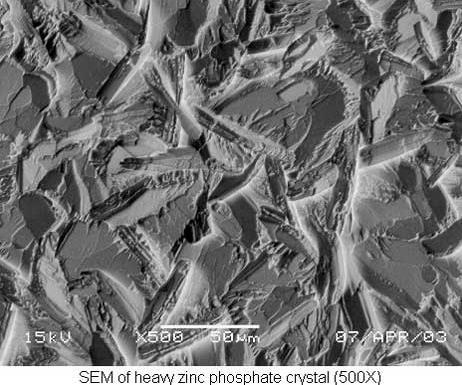
Heavy Zinc Phosphate
Heavy zinc phosphate is usually chosen for its ability to retain rust preventive oils and waxes. The heavy coating, in the range of 1,000–3,000 mg/ft2, acts as an absorbent substrate, holding the rust preventive on the surface of the part. This can provide corrosion protection in excess of 400 hours of 5-percent neutral salt spray exposure, depending on the formulation and concentration of the chosen rust preventive.
Heavy zinc phosphate coatings are applied in an immersion process. Parts are run either on racks or in tumbling barrels. The bath is initially charged at 3–4 percent by volume (30–40 total acid points) and operates at 175–185°F. The bath is controlled with simple titrations that measure concentration (total acid), aggressiveness (free acid) and iron content.
Iron buildup is usually the limiting factor in the service life of the bath. When iron levels become higher than zinc, usually due to high metal throughput, coating quality and uniformity are compromised. At this time, a portion of the bath can be decanted or the bath is charged fresh.
Calcium-modified zinc phosphate
Calcium-modified zinc phosphate is typically used as a base for paint or other organic coatings. The calcium co-deposits with zinc and acts as a built-in grain refiner to form a smooth microcrystalline structure. Coating weights are typically in the range of 150–500 mg/ft2, which allows for enhanced adhesion properties without being as absorptive as a heavy zinc phosphate coating. In addition, the chemical-resistant nature of calcium-modified zinc phosphate coatings confines corrosion to a limited area, often referred to as creepage, if the applied topcoat is damaged. Calcium-modified zinc phosphates are often used prior to rubber bonding and also have wear-resistance.
Calcium-modified zinc phosphate can be applied by spray or immersion. The bath is often a two-component system, with the calcium-rich component added upon start-up and infrequently thereafter. Operating temperature is 150°F for spray and 170–180°F for immersion.
Iron content in the bath may interfere with grain refinement and nonuniform coatings may result. Small but frequent additions of a strong oxidizer will precipitate the iron from the solution, resulting in extended bath life.
Cold-forming zinc phosphates
Cold-forming zinc phosphates are used to facilitate drawing, cold heading, stamping or extruding of the basis metal. These phosphate coatings are designed to retain lubricants under severe conditions of heat and pressure during deformation. The use of the phosphate coating allows increased tool life, faster drawing speeds and more severe reductions of the basis metal.
Coating weights for cold-forming zinc phosphates can range from 500–2,000 mg/ft2. The phosphating solution in this application is operated iron-free to ensure a less abrasive zinc phosphate crystal, which will not scratch dies, score or gall. A strong oxidant, commonly nitrite or chlorate, is needed to drop the iron out of the bath in the form of sludge. In addition, a titanium-based grain refining pre-dip can be used prior to the phosphate to produce a smoother, more uniform crystalline coating.
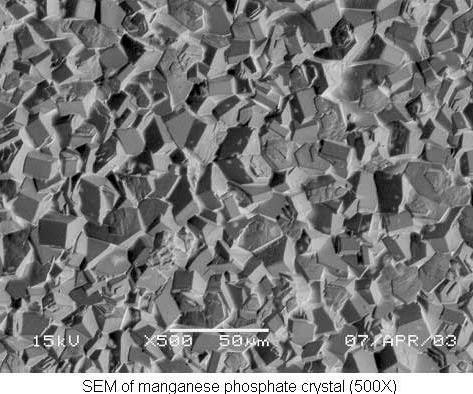
Manganese phosphate
Manganese phosphate is most commonly chosen for its wear-resistant properties. The manganese phosphate coating not only prevents metal-to-metal contact between moving parts, such as cylinder liners, camshafts, piston rings and transmission gears, it also has excellent oil-retentive properties for both lubricity and corrosion resistance.
A grain-refining pre-dip is often used prior to manganese phosphating to ensure a controlled microfinish. A typical manganese phosphate bath is charged at 10 percent by volume and operated at 195–205°F.
Coating weights in excess of 1,500 mg/ft2 are achieved in heavy manganese phosphate baths. Newer formulations allow for lower operating temperatures and less concentration, but coating weight is sacrificed.
The balance of the bath concentration (total acid) when compared with the bath activity (free acid) is critical to control crystal size and coating uniformity. A ratio of 5.5–6:5.1 is recommended and maintained with additions of manganese carbonate. High iron content in the bath can be lowered by treatment with hydrogen peroxide.
Iron phosphate
Iron phosphate is used as a base for paint or powder coat to enhance adhesion. Unlike both zinc and manganese phosphates, in which the metal cation is found in the phosphating solution, the cation of the iron phosphate coating is contributed by the basis metal. The phosphating solution contains alkali metal phosphate and accelerators. Coating weights for iron phosphates range from 25–100 mg/ft2.
Application is usually in a three- or five-stage spray washer, but there are some immersion processes. For three-stage washers, the iron phosphate has an incorporated detergent system to clean and phosphate in one step. In five-stage washers, the cleaning is done separately in stage one, while the phosphate is applied in stage three. A seal is applied in the final stage to minimize under-film corrosion. Many processors are choosing alternatives to hexavalent chrome sealers, substituting organic formulations which are chrome-free.
Iron phosphates are easy to control with a simple titration to determine concentration (1–3 percent by volume) and pH adjustment (4.5–5.5 optimum). Operating temperatures are relatively low (100–130°F).
Developments in the area of phosphate conversion coatings have been aimed at lowering operating temperatures, decreasing sludge volumes produced as a function of the surface area of parts being processed, and adjusting formulations to meet more stringent requirements for coating weights and coating thicknesses. These changes have been mainly to address environmental and economic concerns while at the same time not compromising performance characteristics or quality of resultant coatings.
Phosphate coatings are economical, easy to use, and offer a variety of valuable properties to extend the service life and improve the performance characteristics of the parts being treated.
RELATED CONTENT
-
What is Electrocoating?
E-coat can produce uniform finishes with excellent coverage and outstanding corrosion resistance.
-
Calculating the Cost of Powder Coating
How can you calculate the cost of powder coating a component if you only know its surface area? Powder coating expert Rodger Talbert has the answer.
-
Pretreatment for Painting
Better adhesion, enhanced corrosion and blister resistance, and reduced coating-part interactions make pretreatment a must.