Study Says Metal Orthopedic Implants Unlikely to Trigger Allergy
Despite concerns about cobalt and chromium coating debris on metal hip replacements, noted dermatologist says there is little risk that patients will have a skin reaction or joint pain from the devices.
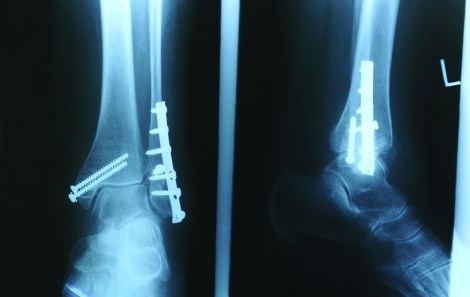
Read more about the subject at http://www.ncbi.nlm.nih.gov/pubmed/20597929
RELATED CONTENT
-
What is the Correct Anodizing Specification?
My company fabricates aluminum and steel pedestrian bridge railing among other bridge parts. We recently got an aluminum railing job that called for “Type I” anodizing per MIL-A-8625. There was no anodic coating thickness called out. We are not anodizers and we are at a loss as to how to write up a meaningful anodizing specification for this railing.
-
How to Apply the 720 Rule to Current Density Anodizing
What can you tell me about the 720 Rule as it applies to current density anodizing? Plating expert Sjon Westre, Ph.D., from Chemeon, answers this question.
-
Fixing Corrosion Between Anodized Aluminum and Steel
Anne Deacon Juhl, Ph.D., with AluConsult, says Galvanic corrosion is due to an electrical contact with a more noble metal or a nonmetallic conductor in a conductive environment.















