Vanadate Conversion Coatings: Alternative to Phosphate?
Emerging pretreatment technology can reduce energy consumption and environmental concerns.
#energy #sustainability
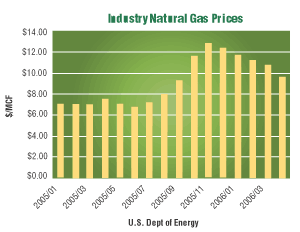
Rising energy costs are one of the top concerns this winter for every metal finishing operation. The U.S. Department of Energy (DoE) projects the cost of industrial natural gas to cost 40% percent more this January than it did in January of 2005. DoE’s forecast predicts costs will decrease in March; however, these costs will still remain higher than prices in March of 2005.
Higher natural gas prices have stoked concern of price increases cascading through the economy, with the most recent monthly inflation gage at 1.2% in September, the largest increase in a quarter century. The United States now has the highest natural gas prices of any industrial country, surpassing those in Germany, the Netherlands and China.
Featured Content
Even worse, officials are warning businesses that they face possible disruptions in the natural gas supply in some states this winter. Under long-established rules, utilities will give the highest priority to supplying natural gas to homes, possibly cutting off some companies and forcing some manufacturers to turn to other energy sources.
It is obvious that metal finishing operations must look for ways to reduce or become less dependent on energy to remain competitive in the global marketplace. But just as important are the issues of continual changes and challenges with environmental compliance. Publicly owned treatment works facilities (POTWs) in certain areas of the country are tightening phosphate discharge limits on metal finishers.
The primary pollutant associated with the eutrophication of our surface waters is phosphorus discharge. Excessive phosphorus discharge causes problems with nuisance algae blooms and reduces water transparency, leaving public waters unsuitable for swimming or other activities.
For example, in February of 2004, the State Legislature of Minnesota and the Minnesota Pollution Control Agency were presented a comprehensive assessment of phosphorus sources in their state. The study’s authors indicated that 46% of non-ingested phosphorus load to the state’s POTWs came from commercial and industrial sources. The study went on to recommend that excise taxes and/or a tax based on effluent strength could reduce this influent source of phosphorus.
Emerging Technologies
During the last year, new metal pretreatment chemical technologies have emerged to help address these concerns. These new inorganic conversion coatings have focused on temperature elimination and/or phosphate discharge reduction.
The majority of these inorganic conversion coatings are fluorozirconium-based. Fluorozirconium coating technology uses complexed transition metal salts to create a thin film on a substrate material similar to a traditional chemical conversion coating. These formulas incorporate the zirconium with other non-regulated transitional metals, and are designed to replace conventional iron phosphating products. In some situations, they can replace zinc phosphating products.
One of these fluorozirconium-based conversion coatings, introduced in 2005, incorporates the transitional metal vanadium. This coating is referred to as vanadate conversion coating.
Vanadium is commonly used as an alloying element in steel. It is found in steels used for making high-quality tools and rust-resistant springs. Vanadium compounds also have been used to enhance the corrosion protection provided by anodized coatings. Small amounts of vanadate added to sulfuric acid (H2SO4) anodizing solutions have been found to make the anodized coating much more protective after hydrothermal sealing.
In other studies, vanadate compounds were used to seal thickened oxide layers on aluminum-based metal-matrix composites by direct application of a vanadate solution. These vanadate-sealed surfaces promoted adhesion of subsequently applied fluoropolymer coatings and resulted in coating systems with very high levels of corrosion resistance in a range of environments.
Vanadate conversion coatings have been developed for use on magnesium alloy substrates in preparation for painting. The coating provides an adherent and corrosion resistant base on magnesium, which is comparable to chromium-based conversion coatings.
Since the late 1990s, vanadium has been used in final seal rinse products as a replacement for chrome-based seal rinses on metal finishing lines processing ferrous and nonferrous substrates. Salt spray performance results with these vanadium-based final seal rinses has improved corrosion resistance over deionized water-only final rinse processes by as much as 300%.
Researchers have theorized that the increased corrosion protection afforded by vanadium-based final rinses is due to vanadium’s tendency to promote the formation of metal ion phosphates, which fill the pores of the phosphate conversion coatings. It also is theorized that the presence of vanadium in a V5+ valence state and of zirconium in the Zr4+ valence state promotes the formation of a zirconium complex, and that deposition of those complexes on the surface of the phosphate conversion coatings provide a corrosion resistance benefit.
In another study examining effects on aluminum alloy substrates, the authors suggested that a particular vanadate conversion coating formula created an inorganic polymerization of V5+, which led to buildup of a film that passivated the surface and inhibited corrosion. Salt-spray testing according to ASTM B 117 suggested that this particular vanadate conversion coating, as compared to chrome conversion coatings, appeared to have self-healing qualities similar to that of chrome conversion coatings on bare aluminum.
Commercial Vanadate Coatings
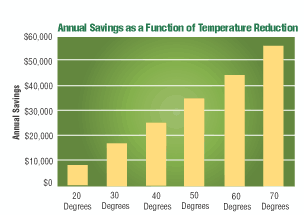
As mentioned earlier, a market-ready vanadate conversion coating product was introduced in 2005 as a pretreatment coating for ferrous and nonferrous substrates. Formulated to replace traditional iron phosphate products, this inorganic vanadate conversion coating product requires no heat to operate. Iron phosphate conversion coating products, on the other hand, can require temperatures of 110–160°F to operate. This vanadate conversion coating treatment also contains no regulated metals, and reduces the amount of phosphate in the process bath to less than 150 ppm. A traditional iron phosphate conversion coating bath can contain phosphate levels in the operating bath in excess of 8,000 ppm.
This ambient inorganic vanadate conversion coating produces an amorphous surface film composed of hydrated vanadium oxides. Under X-ray absorption testing, vanadium oxides and other components of the bath can be detected on ferrous and nonferrous substrates.
Although these fluorozirconium-based conversion coatings provide considerable temperature savings and reduction of phosphate discharge, finishers also gain other benefits. The reaction process of these fluorozirconium-based conversion coatings, which include vanadate conversion coatings, creates very little sludge compared to iron phosphating. Reduced sludge formation results in considerably lower ongoing maintenance costs because fewer bath dumps are needed and the incidence of plugged nozzles and other problems is reduced.
Rinse water consumption is also reduced because of the lower amount of total dissolved solids present in these new coating baths. For example, a metal finishing operation using 5 gpm of overflow to maintain water quality of the rinse stage that follows a traditional iron phosphate bath could reduce that overflow rate by at least 50%. Reduction to approximately 2 gpm has a direct impact on the overall process cost of operating a pretreatment chemical system.
Equipment requirements can vary in regards to these new inorganic conversion coatings. Some fluorozirconium-based conversion coatings require stainless steel equipment and are limited to five-stage pretreatment systems.
Depending on the quality expectations of the metal finisher, the vanadate conversion coating can be used in mild steel equipment. The vanadate conversion coating process can be adapted for spray wand applications, three-stage pretreatment systems, or five-stage systems. A typical vanadate conversion coating process may also include a vanadium-based final seal rinse, which is determined by the quality standards for the particular process.
Performance Results
Fluorozirconium-based treatments for aluminum and final seal rinses have been in existence for some time. Their drawback over the years has been marginal salt spray performance compared to traditional iron phosphate systems using chrome-based final seal rinses. Recent developments with fluorozirconium-based treatments incorporating other elements and/or transition metals, such as vanadium, have produced performance results equal or superior to traditional iron phosphate systems.
| Table I: Vanadate Conversion Coating Comparison | ||||
|
Paint |
Vanadate Conversion Coating | Iron Phosphate Conversion Coating | Hours of Exposure | Substrate |
| Polyester Powder | 0.3 mm creepage from scribe | 4.3 mm creepage from scribe | 504 | CRS |
| Hybrid Powder | 2.5 mm creepage from scribe | 4+ mm creepage from scribe | 504* | CRS |
| Cathodic E-Coat | 0.6 mm creepage from scribe | 3.2 mm creepage from scribe | 504 | CRS |
| Cathodic E-Coat | 0.2 mm creepage from scribe | 6.3 mm creepage from scribe | 1,000 | CRS |
| * Vanadate coating achieved 888 hours. | ||||
The following table provides examples of corrosion resistance performance of a vanadate conversion coating compared to various iron phosphate conversion coatings under a variety of paint systems on cold-rolled steel substrates.
Test panels were prepared and tested in a neutral salt-spray exposure in accordance with ASTM B 117. Ratings were taken of the average creepage failure from the scribe mark after a predetermined number of hours of salt-fog exposure.
In the first example, the vanadate conversion coating was compared to an organic-accelerated iron phosphate conversion coating at an appliance manufacturer. A polyester powder paint was applied to the test panels. The salt spray requirement for this paint process is 504 hours of exposure with creepage from the scribe less than 4.67 mm. As the table indicates, the vanadate conversion coating outperformed the iron phosphate conversion coating.
In the second example, at a manufacturer of ATV components, a hybrid powder was applied over the vanadate conversion coating and compared against an organic accelerated iron phosphate conversion coating. The vanadate conversion coating is applied in a five-stage pretreatment process. The vanadate conversion coating achieved 888 hours of salt fog exposure, whereas the organic accelerated iron phosphate conversion coating failed at 456 hours of salt fog exposure.
The third example is from a manufacturer of HVAC components and systems. The vanadate conversion coating was compared against a chlorate-accelerated iron phosphate conversion coating. Paint is a cathodic e-coat system, and the test was run to 504 hours per the manufacturer’s quality specification. The vanadate conversion coating outperformed the chlorate-accelerated iron phosphate conversion coating in this test.
In the final example, an OEM compared the vanadate conversion coating to a chlorate-accelerated iron phosphate under a cathodic e-coat paint system. The performance of the vanadate conversion coating was tested to ASTM B 117/D 1654 for 1,000 hours of salt spray. In addition, GM9540 Cyclic Scab Corrosion testing was performed for 20 cycles and adhesion testing was conducted to ASTM D 3359. The performance results of the vanadate conversion coating met or exceeded the performance of the traditional iron phosphate.
| Table II: ROI Analysis for Vanadate Conversion Coating | |||||
| Q1 | Q2 | Q3 | Q4 | ||
| Benefits | |||||
| Nozzle/riser maintenance | $480 | $480 | $480 | $480 | |
| Hauling Costs | 0 | $600 | 0 | $600 | |
| Energy savings in Gas |
$2,400 | $2,400 | $2,400 | $2,400 | |
| Quarterly total | $2,880 | $3,480 | $2,880 | $3,480 | |
| Cumulative value | $2,880 | $6,360 | $9,240 | $12,720 | |
| Costs | |||||
| Increased product usage | ($900) | ($900) | ($900) | ($900) | |
| Increased product cost | ($300) | ($300) | ($300) | ($300) | |
| Quarterly total | ($1,200) | ($1,200) | ($1,200) | ($1,200) | |
| Cumulative investment | ($1,200) | ($2,400) | ($3,600) | ($4,800) | |
| Net Value | |||||
| Quarterly total | $1,680 | $2,280 | $1,680 | $2,280 | |
| Cumulative total | $1,680 | $3,960 | $5,640 | $7,920 | |
| 1st year net return | $7,920 | ||||
| Breakeven point | 1st Quarter | ||||
| ROI (1st year) | 165.00% | ||||
Cost Savings/ROI
Taking into account the performance data for vanadate conversion coatings under various paint systems, the next consideration for changing to an ambient fluorozirconium-based conversion coating is the economic benefit derived from reducing the operating temperature of the conversion coating bath. The cost savings and ROI for the metal finishing operation can be significant.
One California-based company compared costs of the vanadate conversion coating against its traditional iron phosphating process based on required maintenance, disposal, and energy costs. Maintenance costs were compared based on eight hours of labor per month to clean nozzles plugged with iron phosphate sludge. Waste hauling cost savings were reduced by 50% in this analysis, and, depending on local conditions, could be eliminated altogether. The manufacturer calculated that heating for its iron phosphate pretreatment system represented 10 to 15% of the plant’s total natural gas costs.
Offsetting the cost benefits in this analysis were the increased product usage and product cost for the vanadate conversion coating.
The break even point in this analysis was achieved after the first quarter; however, the investment is actually spread out over a one-year period. Return on investment is 165% in this analysis, and would vary based on individual differences between various metal finishing operations.
To sum up, the fluorozirconium-based conversion coatings available in today’s marketplace offer an alternative to traditional iron phosphate conversion coatings. The economic and environmental benefits of alternative conversion coatings, such as vanadate, are intriguing, but each metal finishing operation has its own quality requirements and process capabilities. Potential users need to undertake a careful evaluation to determine if the new conversion coatings can offer the level of corrosion resistance performance desired, operate within existing or desired process equipment, and deliver the expected ROI.
Acknowledgments
The author acknowledges the assistance and contributions to this article of Joe Pemberton, John Jandrists, Ken Kaluzny, Scott Schueneman, Dr. Narayan Das and Dr. Jim Ingemanson of the Coral Chemical Co.
RELATED CONTENT
-
Automated Electroplating Systems
Simultaneous engineering reduces energy and resource consumption.
-
Using Alternatives in Pretreatment Baths for Degreasing
Effective cleaning at low, even ambient temperatures, is possible, says Atotech’s Brandon Lloyd, and creates a safer work environment and reduces energy demands.
-
Putting a Lid on Tanks
KCH Engineered Systems says tank covers reduce overall exhaust requirements, fugitive emissions, heat loss and evaporation rates, energy consumption and calculated surface area for exhaust rates.