A Smooth Transition from One Anodizing Process to Another
Knowing when to switch from chromic acid anodizing to thin film sulfuric acid anodizing is important. Learn about why the change should be considered and the challenges in doing so.
#aerospace
Chromic acid anodizing providers are facing increasingly strict regulations in many areas — for example, hexavalent chromium use faces a mandated phase-out in California by 2039. While many anodizers would prefer to continue using the conventional chromic acid anodizing (CAA) process that works well and has worked well for many years, there may be regulatory policy changes in your area requiring you to consider a change. In addition, this type of anodizing is not an easy process, and there are a lot of pitfalls, especially with 2000 series alloys that most anodizer’s customers prefer. The other type of anodizing, thin film sulfuric acid anodizing (TFSAA), is used by some aerospace companies to improve corrosion resistance and act as a base for paint adhesion.
Regardless of the reason for considering a change in your process, the customer still wants an aluminum surface that is flexible, corrosion-resistant and shows good adhesion properties. Therefore, it is important to decide what process to choose from a smart and economical perspective.
The definitions
CAA is mainly used for the protection of critical structures with joints. The corrosion resistance is excellent relative to the thickness of the coating, which usually lies in the range of 0.08 – 0.2 mil. The oxide film is softer and is formed without any significant fatigue loss of the material. The film is easily damaged and is light opaque gray in color. When this film is sealed in a dichromate seal, a greenish color appears.
Featured Content
The process is voltage controlled with a ramping at the beginning of the process, increasing up to 40 volts depending on the type specified. Type I and Type IB are specified in the military specification MIL-PRF-8625F. Type I are conventional coatings produced by about 40 volts, and Type IB are coatings produced by a voltage of 20 to 22.
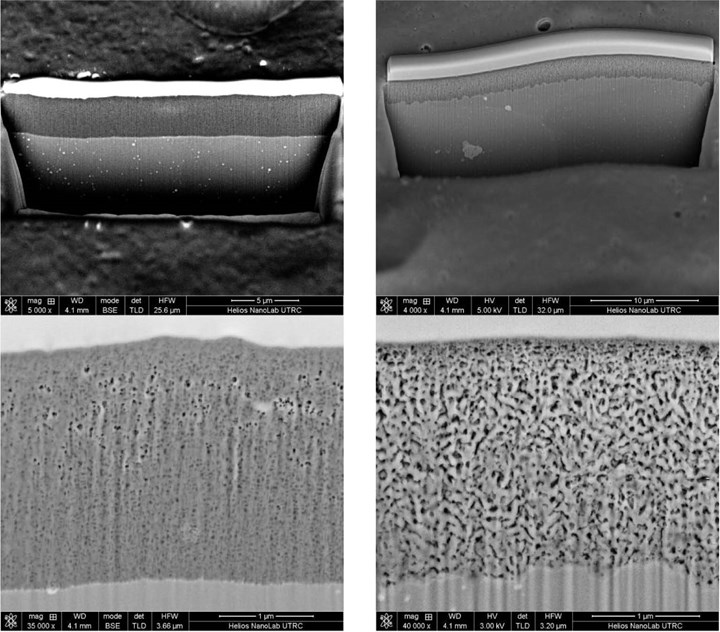
Fig. 1. The FIB-SEM cross-section images at different magnifications for TFSA coatings are on the left and the CAA coatings are on the right, both on EN AW 2024. Photo Credit: all images courtesy of AluConsult
However, TFSAA forms a coating using voltage in a low concentration of sulfuric acid, which leads to a low electrical conductivity and with less chemical attack of the coating. This results in thickness in the same range as CAA films. However, the thickness is more dependent on the anodizing time.
The two coatings have distinct differences in morphologies, as shown in Figure 1. The thickness of the two coatings is about 0.108 mil (2.74 micrometers). The images show a smooth interface between the TFSAA coating and the aluminum substrate (on the left), whereas the CAA coatings show a rougher interface. Both texture and grains in the CAA coating are found to be much coarser than for the TFSAA.
Reasons to make the switch
One of the reasons for anodizers to change from the CAA process to the TFSAA process instead of boric sulfuric acid anodizing (BSAA) is because of the license fee to Boeing. Another reason is the idea of the easy change if they already are running Type II sulfuric acid anodizing — same equipment and process. Unfortunately, this is often a mistake because the two processes vary more than expected.
Having a lower concentration of sulfuric acid gives a less conductive solution, leading to a TFSAA coating with a 33% higher coating density than the CAA coating with comparable thickness (see Figure 1). For dimension and fatigue- sensitive applications, this variation can be critical. Controlling coating thickness is critical for both corrosion and mechanical properties, but also the morphology of the oxide is of great importance. This is true for Type II, also, where variation in the oxide structure leads to variations in color and properties.
CAA replacement
Besides Type I and Type IB, other processes are available, such as Type IC, with BSAA as the NADCAP-approved process. Type IIB — thin sulfuric acid anodizing — is the other alternative for the replacement of chromic acid. This process can also be named Type IC because the type is classified by non-chromic acid anodizing used as an alternative for Type I and Type IB.
The confusion is obvious, and according to MIL-PRF-8625, the difference can be found in the coating weight. Type IC has a coating weight of 200 – 700 mg/ft2, whereas Type IIB enables coating weight up to 1,000 mg/ft2 as a maximum.
For the fatigue-sensitive parts, the BSAA has long been the only fully implemented alternative to CAA, according to Naval Air Systems Command (NAVAIR). The TFSAA is an alternative, also, and new improvements enhance the TFSAA process. It meets the process control test of MIL-PRF-8625F but has still not been fully implemented in all specifications.
Challenges
Measuring thicknesses below 0.28 mil (7 micrometers) is always tricky and critical because the deviation in the measuring device is in the range of the layer thickness that needs to be measured. Therefore, Type IIB is defined
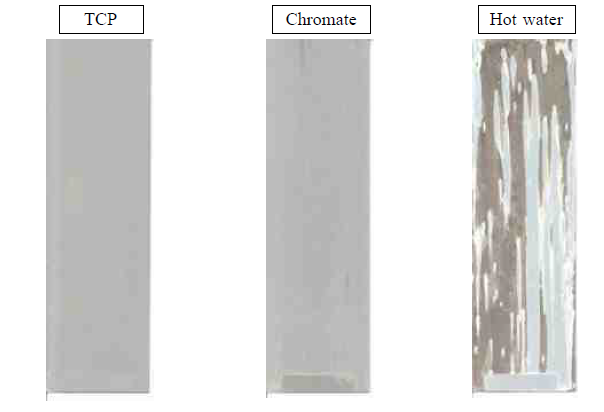
Fig. 2. This shows TFSAA with various sealers on 2024-T3 aluminum after exposure to 1,000 hours of ASTM B 117 salt spray.
more by coating weight than by thickness compared to Type II anodizing, where thickness is the preferred test and often the only control done.
The sealing process is another deviation between the two sulfuric anodizing processes because the hot water sealing process is insufficient to pass the corrosion test when using a TFSAA coating (see Figure 2).
Some of the programs for aerospace call out the low chrome concentration seal with less than 0.02 ounce per gallon. This sealing solution can be difficult to maintain and control compared to the conventional 5% sodium dichromate seal.
In Figure 2, it is obvious that the TFSAA still needs a sealer with chrome to pass the corrosion resistance test, as the conventional hot water sealing is insufficient.
The porous layer formed and sealed with the low concentration of sodium dichromate does not have enough active chromate, and the corrosion inhibitor is the layer to protect against corrosion. Therefore, these layers are often non-conformant to the MIL-PRF-8625. Many anodizers have a dichromate seal in their process line but often, this is the 5% dichromate seal and therefore, a new process tank must be included with new procedures in place.
These are only some of the deviations anodizers face when changing from chromic acid anodizing. To transition well, time and openness to change must be invested. Sometimes, more tests and changes must be performed to prepare for the transition.
About the Author
Anne Deacon Juhl
Anne Deacon Juhl has a Ph.D. in pulse anodizing. During her studies, she established a personal network in Europe, the U.S., and Scandinavia. As a consultant within the field of aluminum finishing, she has worked with many kinds of chemical and electrochemical surface treatments, such as anodizing, coatings, electroplating, and electroless plating, with 25 years of experience in the industry.
RELATED CONTENT
-
Chicago Anodizer Emphasizes Relationships as Key to Growing Business
Chicago Anodizing Company continues to grow its 75-year old business by investing in its people and partners, and by embracing new technologies.
-
Top Shop Aces Outstanding Customer Service
More than a finishing shop, this anodizing, powder coating and vacuum resin impregnating business goes above and beyond for its customers by being a resource for whatever their finishing needs might demand.
-
Understanding and Managing White Spots on Anodized Aluminum
Having trouble with spotting defects when anodizing? Taj Patel of Techevon LLC offers a helpful overview of the various causes of white spots and potential solutions.