Electrodeposition and Characterization of Nickel–Titania Nanocomposite Coatings from Gluconate Baths
Ni-TiO2 nanocomposites with varying titania content were electrodeposited on copper substrates employing a gluconate electrolyte. The changes in microstructure and corrosion behavior of electrodeposited nickel with respect to titania addition were studied.
A survey of the literature shows that gluconate electrolytes have been used to electroplate metals such as nickel,20 copper,21 tin22 and zinc.23 Organic additives have been found to affect the electrothrowing power of nickel from sulfate, chloride and Watts baths. The objective of the present study is to electrodeposit a nickel-titania composite from a gluconate bath in order to determine the dependence of coating characteristics on several electroplating variables and to characterize the coatings by SEM, EDX and XRD and examining their hardness and corrosion resistance.
Experimental
Featured Content
The plating bath used (described in Table 1) was freshly prepared from reagent grade chemicals and doubly distilled water. Copper sheet cathodes and pure nickel sheet anodes, both of dimensions 2×2 cm2 were used. The copper sheet cathodes were mechanically polished with different grade emery papers and then immersed in a pickling solution (300 mL H2SO4 + 100 mL HNO3 + 5 mL HCl + 595 mL doubly-distilled water) for 1 min, washed with distilled water, rinsed with acetone, dried and finally weighed. The pH was measured using Microprocessor pH/mV/°C Meter (Model CP 5943-45USA) and adjusted with a 20% NaOH solution. The temperature was controlled by using hot plate-magnetic stirrer (Philip Harris Ltd). Direct current was supplied by a DC power supply unit (GP-4303D). The copper cathodes were weighed before and after electrodeposition for a certain period of time and at fixed current density. From the change in weights of the cathodes, the deposited weight was calculated. The composition was determined using the following procedure:
- The coating layer was stripped using 10% H2SO4 solution. The next step was to use it as an anode in an electroplating cell by which the coating layer was dissolved in the solution, which was then diluted to 250 mL with doubly-distilled water.
- Analysis was done using atomic absorption spectrophotometry (Perkin-Elmer 3100, Germany).
- The solution obtained was further diluted by dissolving 5 mL in doubly-distilled water to 250 mL.
- Nickel standard solutions for the elements to be detected were prepared (1 g Ni metal in (1+1) HNO3, diluted to 1 L with 1 vol% HNO3), Ni, air-acetylene flame gases, wavelength of 232 nm.
- The titania weight was calculated by subtracting the obtained nickel weight from the total deposit weight.
The results were confirmed for some samples with EDX analysis.
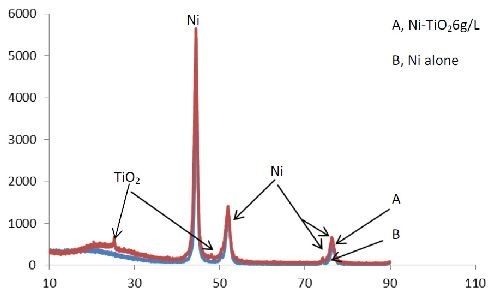
The surface of the as-deposited nickel and nickel-titania was morphologically inspected using scanning electron microscopy (SEM; JEOL-5410 attached to an EDX unit). The phases of the surface and phase changes of the different coated substrates were investigated by using an x-ray diffractometer (Broker AXS-D8 X-ray diffractometer, Advance, Germany), with a copper target (Cuλ = 1.54060 Å) and a nickel filter. The coating thickness was measured by taking a cross-section of the coated layer using a coating thickness/Neophot2-optical microscope (Germany). The Vickers microhardness of the deposits was measured under a 50-g load on the specimen material by using a Shimdzu Hardness tester. The electrochemical experiments were performed using a Volta Lab 40 (Model PGZ301) with the aid of commercial software (Volta Master 4 version 7.08). A saturated calomel electrode (SCE) and a platinized platinum black were used as the reference and auxiliary electrodes, respectively, with the various plated samples used as the working electrode. The electrochemical cell was filled with a 3.5% NaCl electrolyte. Volta Master 4 calculates and displays the corrosion rate, Rcorr, in µm/year. This rate is calculated from the measured corrosion current density, icorr, the density D, the atomic mass M and the valence V entered in the Tafel dialogue box. The calculation is performed as follows:
Corrosion rate (µm/yr) = [icorr (A/cm2) × M (g)] / [D (g/cm3) × V] × 3270
Table 1 - The bath composition and operating conditions of nickel-titania composite process.
Nickel sulfate | 0.2 mol/L |
Boric acid | 0.4 mol/L |
Ammonium sulfate | 0.4 mol/L |
Sodium gluconate | 0.2 mol/L |
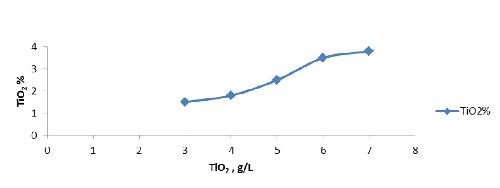
Titania (50 nm) | 3 - 7 g/L |
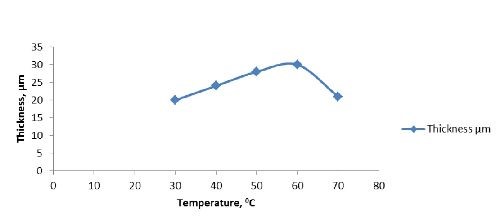
Temperature | 30 - 70°C |
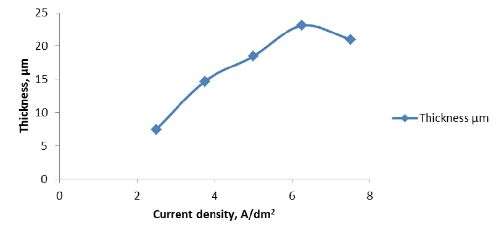
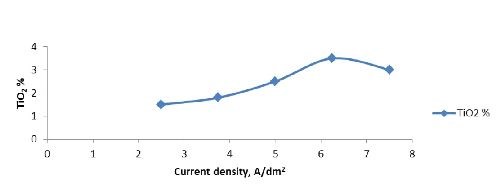
Current density | 2.5 - 7.5 A/dm2 |
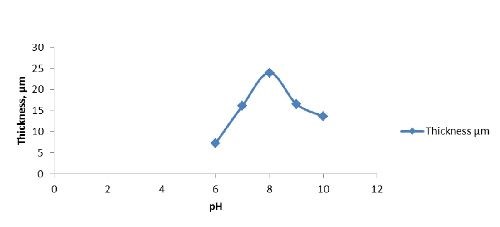
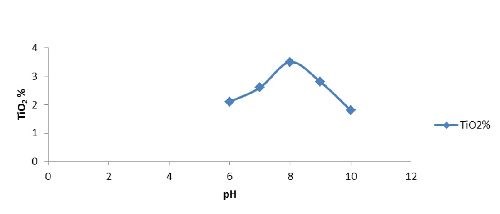
pH | 6 - 10 |
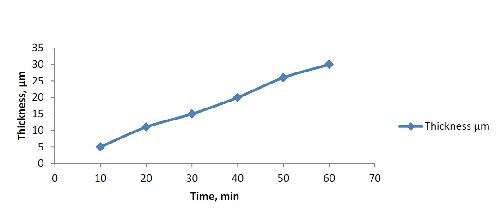
Time | 10 - 60 min |
Stirring speed | 150 rpm |
The same bath composition was used for nickel electrodeposition without titania for comparison.
Results and discussion
Optimization of nickel-titania electrodeposition parameters:
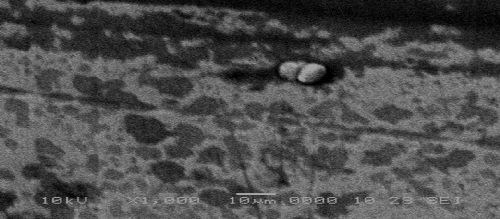
The addition of titania nanoparticles to the bath obviously affects the surface morphology of the coatings, as shown in Figs. 11 and 12. The titania particles appear as light spots in the darker nickel matrix. Because of the addition of TiO2 nanoparticles to the bath, the microstructure of the nickel matrix changed from spherical (Fig. 11) to granular (Fig. 12).
Microhardness
The incorporation of TiO2 in the coating increased the hardness. In the case of 3.5 wt% TiO2, the observed hardness was 440HV50, instead of 340HV50 in the case of the pure nickel coating.
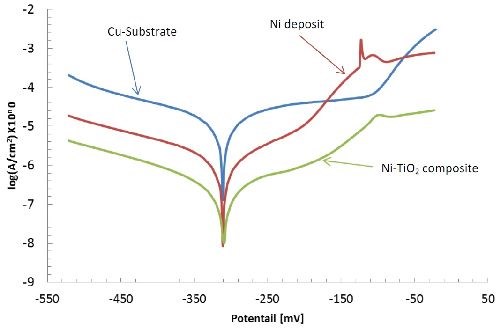
Ecorr (i=0), mV | icorr, µA/cm2 | Rp, kΩ•cm2 | Beta a, mV | Beta c, mV | Corr. rate, µm/yr | |
Cu substrate | -315.0 | 6.2359 | 1.93 | 73.4 | -82.7 | 72.40 |
Ni-gluconate | -228.7 | 0.5479 | 17.83 | 64.3 | -66.2 | 6.362 |
Ni-TiO2 | -264.3 | 120.11 | 100.77 | 66.5 | -78.8 | 1.394 |
RELATED CONTENT
-
Nickel Electroplating
Applications, plating solutions, brighteners, good operating practices and troubleshooting.
-
Zinc Electroplating
Choosing the best process for your operation.
-
Copper Plating on Aluminum and Aluminum Alloys
How can I plate copper on aluminum?