Inconsistent Gloss Problem
Question: Regarding the letter from T.B. in the October Painting Clinic, I had run across a similar fouling in the oven that would cause an inconsistent gloss.
Question:
Regarding the letter from T.B. in the October Painting Clinic, I had run across a similar fouling in the oven that would cause an inconsistent gloss. Ammonium carbonate took care of the problem. Of course, we would usually investigate the source of the material causing the problem, usually triclor or perchlor somewhere in the plant. When we could not find the source, we used the ammonium carbonate. S.B.
Answer:
This question refers to a problem relating to inconsistent gloss on parts coated using a two-component polyurethane baking enamel. T.B. wanted a chart showing catalyst ounces vs. gloss. I gave him chapter and verse on handling two-component materials as well as typical causes for gloss variations. As usual, I left the door open for remarks from my faithful readers (without them, there would be no Painting Clinic).
Featured Content
If S.B. said that he used ammonium carbonate to cure a fouled oven and maintain gloss consistency, I believe him. However, as a scientist, my curiosity was aroused. I wondered what the effect was. How did S.B. use it, and in what form? Naturally I turned to my freshman chemistry textbook, "College Chemistry, An Introductory Textbook of General Chemistry," Linus Pauling, W. H. Freeman and Company, 1950. I knew that I was in trouble when I realized its Periodic Table showed only 98 elements. There was nothing about ammonium carbonate mentioned. I went through my archives of coatings and scientific books without any references to that compound. My search was not in vain. I did re-learn how a coke byproducts oven works. I just built such an oven battery for my HO scale railroad.
Finally, I checked my "Random House Dictionary of the English Language," Random House, Inc., 1973, an 11-lb volume with a Periodic Table listing 103 elements. It defines ammonium carbonate as "a water-soluble mixture of ammonium bicarbonate and ammonium carbamate,...used chiefly in the manufacture of smelling salts and baking powder." Thanks again for your help, S.B. After I'm revived by the smelling salts, I will bake you a cake.
RELATED CONTENT
-
Painting Over Powder Coating
How safely can they apply their wet paint over our powder coated parts?
-
Curing Oven Basics
Simply heating up the substrate does not cure the coating. There are many variables to consider when choosing the best cure oven for your application...
-
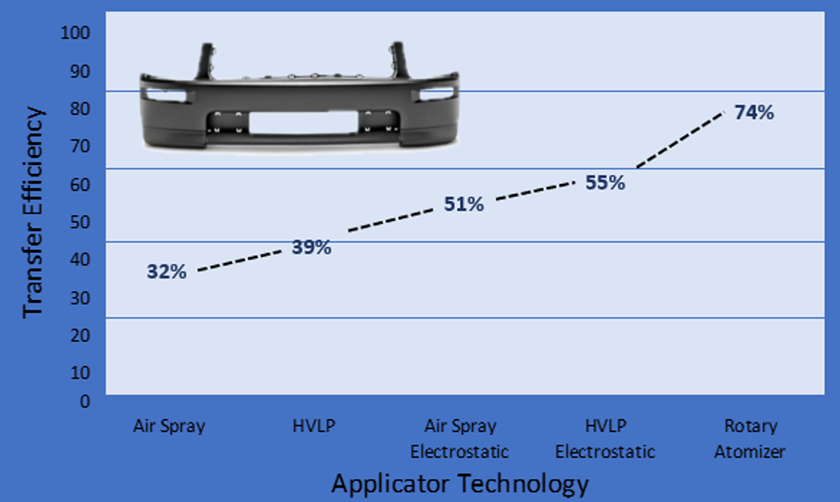
Improving Transfer Efficiencies in Coating Operations
There are many methods for addressing electrostatic grounding in metal painting processes, and Tim Ulshafer from Mueller Electric says the best method for your process is a simple and worthwhile exercise.